当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New Knowledge-Based Scoring Function with Inclusion of Backbone Conformational Entropies from Protein Structures
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acs.jcim.7b00601 Xinxiang Wang 1 , Di Zhang 1 , Sheng-You Huang 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1021/acs.jcim.7b00601 Xinxiang Wang 1 , Di Zhang 1 , Sheng-You Huang 1
Affiliation
|
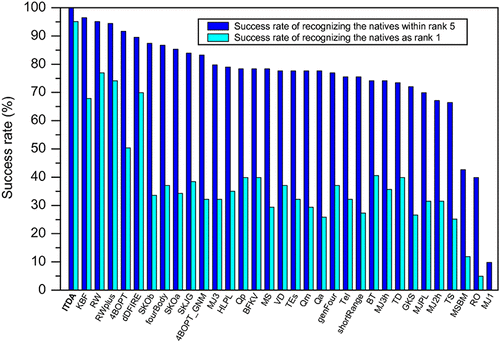
Accurate prediction of a protein’s structure requires a reliable free energy function that consists of both enthalpic and entropic contributions. Although considerable progresses have been made in the calculation of potential energies in protein structure prediction, the computation for entropies of protein has lagged far behind, due to the challenge that estimation of entropies often requires expensive conformational sampling. In this study, we have used a knowledge-based approach to estimate the backbone conformational entropies from experimentally determined structures. Instead of conducting computationally expensive MD/MC simulations, we obtained the entropies of protein structures based on the normalized probability distributions of back dihedral angles observed in the native structures. Our new knowledge-based scoring function with inclusion of the backbone entropies, which is referred to as ITScoreDA or ITDA, was extensively evaluated on 16 commonly used decoy sets and compared with 50 other published scoring functions. It was shown that ITDA is significantly superior to the other tested scoring functions in selecting native structures from decoys. The present study suggests the role of backbone conformational entropies in protein structures and provides a way for fast estimation of the entropic effect.
中文翻译:

基于知识的新计分功能,包括蛋白质结构中的骨架构象熵
准确预测蛋白质的结构需要可靠的自由能函数,该函数应包含焓和熵。尽管在蛋白质结构预测中的势能计算方面已经取得了很大进展,但是由于熵估计常常需要昂贵的构象采样这一挑战,蛋白质熵的计算已经远远落后了。在这项研究中,我们使用了一种基于知识的方法来根据实验确定的结构估算骨架构象熵。代替进行计算上昂贵的MD / MC模拟,我们基于在天然结构中观察到的反二面角的归一化概率分布,获得了蛋白质结构的熵。我们对新的基于知识的评分功能(包括ITScoreDA或ITDA)包含骨干熵,对16种常用诱饵集进行了广泛评估,并将其与其他50种发布的评分功能进行了比较。结果表明,ITDA在从诱饵中选择天然结构方面显着优于其他经过测试的评分功能。本研究表明骨架构象熵在蛋白质结构中的作用,并提供了一种快速估计熵效应的方法。
更新日期:2018-02-14
中文翻译:

基于知识的新计分功能,包括蛋白质结构中的骨架构象熵
准确预测蛋白质的结构需要可靠的自由能函数,该函数应包含焓和熵。尽管在蛋白质结构预测中的势能计算方面已经取得了很大进展,但是由于熵估计常常需要昂贵的构象采样这一挑战,蛋白质熵的计算已经远远落后了。在这项研究中,我们使用了一种基于知识的方法来根据实验确定的结构估算骨架构象熵。代替进行计算上昂贵的MD / MC模拟,我们基于在天然结构中观察到的反二面角的归一化概率分布,获得了蛋白质结构的熵。我们对新的基于知识的评分功能(包括ITScoreDA或ITDA)包含骨干熵,对16种常用诱饵集进行了广泛评估,并将其与其他50种发布的评分功能进行了比较。结果表明,ITDA在从诱饵中选择天然结构方面显着优于其他经过测试的评分功能。本研究表明骨架构象熵在蛋白质结构中的作用,并提供了一种快速估计熵效应的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号