当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organocatalytic C−F Bond Activation with Alanes
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-03-30 , DOI: 10.1002/chem.201706061 Alma D. Jaeger 1 , Christian Ehm 2 , Dieter Lentz 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-03-30 , DOI: 10.1002/chem.201706061 Alma D. Jaeger 1 , Christian Ehm 2 , Dieter Lentz 1
Affiliation
|
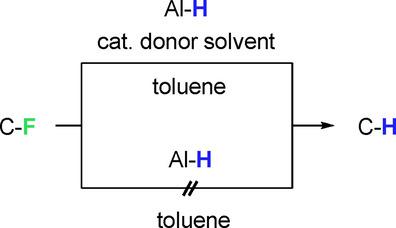
Hydrodefluorination reactions (HDF) of per‐ and polyfluorinated olefins and arenes by cheap aluminum alkyl hydrides in non‐coordinating solvents can be catalyzed by O and N donors. TONs with respect to the organocatalysts of up to 87 have been observed. Depending on substrate and concentration, high selectivities can be achieved. For the prototypical hexafluoropropene, however, low selectivities are observed (E/Z≈2). DFT studies show that the preferred HDF mechanism for this substrate in the presence of donor solvents proceeds from the dimer Me4Al2(μ‐H)2⋅THF by nucleophilic vinylic substitution (SNV)‐like transition states with low selectivity and without formation of an intermediate, not via hydrometallation or σ‐bond metathesis. In the absence of donor solvents, hydrometallation is preferred but this is associated with inaccessibly high activation barriers at low temperatures. Donor solvents activate the aluminum hydride bond, lower the barrier for HDF significantly, and switch the product preference from Z to E. The exact nature of the donor has only a minimal influence on the selectivity at low concentrations, as the donor is located far away from the active center in the transition states. The mechanism changes at higher donor concentrations and proceeds from Me2AlH⋅THF via SNV and formation of a stable intermediate, from which elimination is unselective, which results in a loss of selectivity.
中文翻译:

烷烃的有机催化CF键活化
O和N供体可催化廉价的烷基氢化铝在非配位溶剂中对全氟和多氟烯烃和芳烃进行加氢脱氟反应(HDF)。已经观察到相对于多达87种有机催化剂的TON。取决于底物和浓度,可以实现高选择性。对于典型的六氟丙烯,但是,低选择性观察到(É / Ž ≈2)。DFT的研究表明,优选的HDF机制此基片以供体的溶剂进行的从二聚体Me中存在4的Al 2(μ-H)2 ⋅通过亲核取代乙烯基THF(S ÑV)样过渡态,选择性低且没有形成中间体,这不是通过加氢金属化或σ键复分解实现的。在不存在供体溶剂的情况下,优选加氢金属化,但这与低温下难以获得的高活化势垒有关。供体溶剂激活氢化铝键,显著降低为HDF的屏障,并切换产品偏好从Ž到È。供体的确切性质对低浓度下的选择性仅具有最小的影响,因为在过渡态中供体远离活性中心。在较高的供体的浓度,并进入与Me机制变化2的AlH ⋅ THF通过S ÑV和形成稳定的中间体,从中除去是非选择性的,这导致选择性的损失。
更新日期:2018-03-30
中文翻译:

烷烃的有机催化CF键活化
O和N供体可催化廉价的烷基氢化铝在非配位溶剂中对全氟和多氟烯烃和芳烃进行加氢脱氟反应(HDF)。已经观察到相对于多达87种有机催化剂的TON。取决于底物和浓度,可以实现高选择性。对于典型的六氟丙烯,但是,低选择性观察到(É / Ž ≈2)。DFT的研究表明,优选的HDF机制此基片以供体的溶剂进行的从二聚体Me中存在4的Al 2(μ-H)2 ⋅通过亲核取代乙烯基THF(S ÑV)样过渡态,选择性低且没有形成中间体,这不是通过加氢金属化或σ键复分解实现的。在不存在供体溶剂的情况下,优选加氢金属化,但这与低温下难以获得的高活化势垒有关。供体溶剂激活氢化铝键,显著降低为HDF的屏障,并切换产品偏好从Ž到È。供体的确切性质对低浓度下的选择性仅具有最小的影响,因为在过渡态中供体远离活性中心。在较高的供体的浓度,并进入与Me机制变化2的AlH ⋅ THF通过S ÑV和形成稳定的中间体,从中除去是非选择性的,这导致选择性的损失。


























 京公网安备 11010802027423号
京公网安备 11010802027423号