当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N‐Acyl‐Glutarimides: Privileged Scaffolds in Amide N–C Bond Cross‐Coupling
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-03-24 , DOI: 10.1002/ejoc.201800109 Guangrong Meng 1 , Michal Szostak 1
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-03-24 , DOI: 10.1002/ejoc.201800109 Guangrong Meng 1 , Michal Szostak 1
Affiliation
|
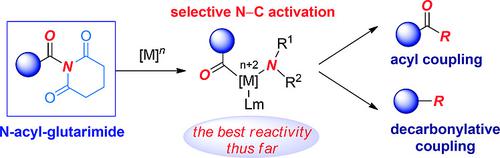
In this Microreview, we describe the recent exciting developments in the burgeoning area of amide N–C cross‐coupling enabled by amide bond twist of N‐acyl‐glutarimides. Since the initial reports in 2015, these amides have been demonstrated to be by far the most reactive amide derivatives in the biologically‐relevant manifold of N–C activation/cross‐coupling, thus stimulating the development of more than 10 previously unknown catalytic modes of reactivity of the amide bond. The capacity of N‐acyl‐glutarimides as privileged scaffolds to expedite acyl and decarbonylative cross‐couplings by amide N–C cleavage is discussed.
中文翻译:

N-酰基-戊二酰亚胺:酰胺N-C键交叉偶联中的特权支架
在本Microreview中,我们描述了由于N-酰基-戊二酰胺的酰胺键加捻而使酰胺N-C交叉偶联迅速发展的最新领域。自2015年首次报告以来,已证明这些酰胺是迄今为止与N-C活化/交叉偶联生物学相关的生物活性最高的酰胺衍生物,从而刺激了10多种以前未知的催化方式的发展。酰胺键的反应性。讨论了N-酰基戊二酰亚胺作为特有支架通过酰胺N-C裂解加速酰基和脱羰基交叉偶联的能力。
更新日期:2018-03-24
中文翻译:

N-酰基-戊二酰亚胺:酰胺N-C键交叉偶联中的特权支架
在本Microreview中,我们描述了由于N-酰基-戊二酰胺的酰胺键加捻而使酰胺N-C交叉偶联迅速发展的最新领域。自2015年首次报告以来,已证明这些酰胺是迄今为止与N-C活化/交叉偶联生物学相关的生物活性最高的酰胺衍生物,从而刺激了10多种以前未知的催化方式的发展。酰胺键的反应性。讨论了N-酰基戊二酰亚胺作为特有支架通过酰胺N-C裂解加速酰基和脱羰基交叉偶联的能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号