当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
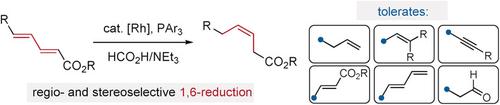
Chemoselective Synthesis of Z‐Olefins through Rh‐Catalyzed Formate‐Mediated 1,6‐Reduction
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-12 , DOI: 10.1002/anie.201800361 Raphael Dada 1 , Zhongyu Wei 1 , Ruohua Gui 1 , Rylan J. Lundgren 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-12 , DOI: 10.1002/anie.201800361 Raphael Dada 1 , Zhongyu Wei 1 , Ruohua Gui 1 , Rylan J. Lundgren 1
Affiliation
|
Z‐olefins are important functional units in synthetic chemistry; their preparation has thus received considerable attention. Many prevailing methods for cis‐olefination are complicated by the presence of multiple unsaturated units or electrophilic functional groups. In this study, Z‐olefins are delivered through selective reduction of activated dienes using formic acid. The reaction proceeds with high regio‐ and stereoselectivity (typically >90:10 and >95:5, respectively) and preserves other alkenyl, alkynyl, protic, and electrophilic groups.
中文翻译:

通过Rh催化的甲酰胺介导的1,6-还原化学合成Z-烯烃
Z烯烃是合成化学中的重要功能单元;因此,他们的准备受到了极大的关注。多个不饱和单元或亲电子官能团的存在使许多流行的顺式烯烃聚合方法变得复杂。在这项研究中,Z-烯烃是通过使用甲酸选择性还原活化的二烯而得到的。反应进行时具有很高的区域选择性和立体选择性(通常分别> 90:10和> 95:5),并保留了其他烯基,炔基,质子和亲电基团。
更新日期:2018-03-12
中文翻译:

通过Rh催化的甲酰胺介导的1,6-还原化学合成Z-烯烃
Z烯烃是合成化学中的重要功能单元;因此,他们的准备受到了极大的关注。多个不饱和单元或亲电子官能团的存在使许多流行的顺式烯烃聚合方法变得复杂。在这项研究中,Z-烯烃是通过使用甲酸选择性还原活化的二烯而得到的。反应进行时具有很高的区域选择性和立体选择性(通常分别> 90:10和> 95:5),并保留了其他烯基,炔基,质子和亲电基团。



























 京公网安备 11010802027423号
京公网安备 11010802027423号