Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Citrate stabilized gold nanoparticles interfere with amyloid fibril formation: D76N and ΔN6 β2-microglobulin variants†
Nanoscale ( IF 6.7 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1039/c7nr06808e Giorgia Brancolini 1, 2, 3, 4 , Maria Celeste Maschio 1, 2, 3, 4 , Cristina Cantarutti 4, 5, 6, 7 , Alessandra Corazza 4, 5, 6, 7, 8 , Federico Fogolari 4, 5, 6, 7, 8 , Vittorio Bellotti 4, 8, 9, 10, 11 , Stefano Corni 2, 12, 13, 14, 15 , Gennaro Esposito 1, 2, 3, 4, 8
Nanoscale ( IF 6.7 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1039/c7nr06808e Giorgia Brancolini 1, 2, 3, 4 , Maria Celeste Maschio 1, 2, 3, 4 , Cristina Cantarutti 4, 5, 6, 7 , Alessandra Corazza 4, 5, 6, 7, 8 , Federico Fogolari 4, 5, 6, 7, 8 , Vittorio Bellotti 4, 8, 9, 10, 11 , Stefano Corni 2, 12, 13, 14, 15 , Gennaro Esposito 1, 2, 3, 4, 8
Affiliation
|
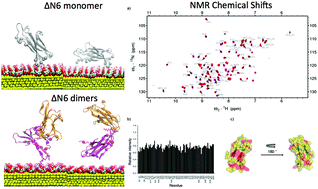
Protein aggregation including the formation of dimers and multimers in solution, underlies an array of human diseases such as systemic amyloidosis which is a fatal disease caused by misfolding of native globular proteins damaging the structure and function of affected organs. Different kind of interactors can interfere with the formation of protein dimers and multimers in solution. A very special class of interactors are nanoparticles thanks to the extremely efficient extension of their interaction surface. In particular citrate-coated gold nanoparticles (cit-AuNPs) were recently investigated with amyloidogenic protein β2-microglobulin (β2m). Here we present the computational studies on two challenging models known for their enhanced amyloidogenic propensity, namely ΔN6 and D76N β2m naturally occurring variants, and disclose the role of cit-AuNPs on their fibrillogenesis. The proposed interaction mechanism lies in the interference of the cit-AuNPs with the protein dimers at the early stages of aggregation, that induces dimer disassembling. As a consequence, natural fibril formation can be inhibited. Relying on the comparison between atomistic simulations at multiple levels (enhanced sampling molecular dynamics and Brownian dynamics) and protein structural characterisation by NMR, we demonstrate that the cit-AuNPs interactors are able to inhibit protein dimer assembling. As a consequence, the natural fibril formation is also inhibited, as found in experiment.
中文翻译:

柠檬酸盐稳定的金纳米颗粒会干扰淀粉样蛋白原纤维的形成:D76N和ΔN6β2-微球蛋白变体†
蛋白质的聚集包括溶液中二聚体和多聚体的形成,是一系列人类疾病的基础,例如系统性淀粉样变性病,这是一种致命疾病,由天然球状蛋白质的错误折叠引起,破坏了受影响器官的结构和功能。不同种类的相互作用物可干扰溶液中蛋白质二聚体和多聚体的形成。一类非常特殊的相互作用剂是纳米颗粒,这要归功于其相互作用表面的极高效扩展。特别是柠檬酸盐-包被的金颗粒(CIT-的AuNP)最近与成淀粉样蛋白β2微球蛋白研究(β 2 M)。在这里,我们提出两个计算研究其增强淀粉样蛋白的倾向,即ΔN6和D76Nβ称为挑战模式2天然存在的变体,并揭示了cit-AuNPs在其原纤维形成中的作用。所提出的相互作用机制在于在聚合的早期阶段,cit-AuNPs对蛋白质二聚体的干扰会导致二聚体的分解。结果,可以抑制天然原纤维形成。依靠多级原子模拟(增强的采样分子动力学和布朗动力学)之间的比较和NMR进行的蛋白质结构表征,我们证明了cit-AuNPs相互作用子能够抑制蛋白质二聚体组装。结果,如在实验中发现的,天然原纤维的形成也被抑制。
更新日期:2018-02-14
中文翻译:

柠檬酸盐稳定的金纳米颗粒会干扰淀粉样蛋白原纤维的形成:D76N和ΔN6β2-微球蛋白变体†
蛋白质的聚集包括溶液中二聚体和多聚体的形成,是一系列人类疾病的基础,例如系统性淀粉样变性病,这是一种致命疾病,由天然球状蛋白质的错误折叠引起,破坏了受影响器官的结构和功能。不同种类的相互作用物可干扰溶液中蛋白质二聚体和多聚体的形成。一类非常特殊的相互作用剂是纳米颗粒,这要归功于其相互作用表面的极高效扩展。特别是柠檬酸盐-包被的金颗粒(CIT-的AuNP)最近与成淀粉样蛋白β2微球蛋白研究(β 2 M)。在这里,我们提出两个计算研究其增强淀粉样蛋白的倾向,即ΔN6和D76Nβ称为挑战模式2天然存在的变体,并揭示了cit-AuNPs在其原纤维形成中的作用。所提出的相互作用机制在于在聚合的早期阶段,cit-AuNPs对蛋白质二聚体的干扰会导致二聚体的分解。结果,可以抑制天然原纤维形成。依靠多级原子模拟(增强的采样分子动力学和布朗动力学)之间的比较和NMR进行的蛋白质结构表征,我们证明了cit-AuNPs相互作用子能够抑制蛋白质二聚体组装。结果,如在实验中发现的,天然原纤维的形成也被抑制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号