当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigation of excited state, reductive quenching, and intramolecular electron transfer of Ru(ii)–Re(i) supramolecular photocatalysts for CO2 reduction using time-resolved IR measurements†
Chemical Science ( IF 8.4 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1039/c7sc05338j Kazuhide Koike 1 , David C Grills 2 , Yusuke Tamaki 3 , Etsuko Fujita 2 , Kei Okubo 3 , Yasuomi Yamazaki 3 , Masaki Saigo 4 , Tatsuhiko Mukuta 3 , Ken Onda 4 , Osamu Ishitani 3
Chemical Science ( IF 8.4 ) Pub Date : 2018-02-14 00:00:00 , DOI: 10.1039/c7sc05338j Kazuhide Koike 1 , David C Grills 2 , Yusuke Tamaki 3 , Etsuko Fujita 2 , Kei Okubo 3 , Yasuomi Yamazaki 3 , Masaki Saigo 4 , Tatsuhiko Mukuta 3 , Ken Onda 4 , Osamu Ishitani 3
Affiliation
|
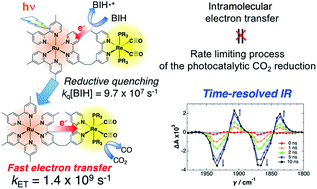
Supramolecular photocatalysts in which Ru(II) photosensitizer and Re(I) catalyst units are connected to each other by an ethylene linker are among the best known, most effective and durable photocatalytic systems for CO2 reduction. In this paper we report, for the first time, time-resolved infrared (TRIR) spectra of three of these binuclear complexes to uncover why the catalysts function so efficiently. Selective excitation of the Ru unit with a 532 nm laser pulse induces slow intramolecular electron transfer from the 3MLCT excited state of the Ru unit to the Re unit, with rate constants of (1.0–1.1) × 104 s−1 as a major component and (3.5–4.3) × 106 s−1 as a minor component, in acetonitrile. The produced charge-separated state has a long lifetime, with charge recombination rate constants of only (6.5–8.4) × 104 s−1. Thus, although it has a large driving force (−ΔG0CR ∼ 2.6 eV), this process is in the Marcus inverted region. On the other hand, in the presence of 1-benzyl-1,4-dihydronicotinamide (BNAH), reductive quenching of the excited Ru unit proceeds much faster (kq[BNAH (0.2 M)] = (3.5–3.8) × 106 s−1) than the abovementioned intramolecular oxidative quenching, producing the one-electron-reduced species (OERS) of the Ru unit. Nanosecond TRIR data clearly show that intramolecular electron transfer from the OERS of the Ru unit to the Re unit (kET > 2 × 107 s−1) is much faster than from the excited state of the Ru unit, and that it is also faster than the reductive quenching process of the excited Ru unit by BNAH. To measure the exact value of kET, picosecond TRIR spectroscopy and a stronger reductant were used. Thus, in the case of the binuclear complex with tri(p-fluorophenyl)phosphine ligands (RuRe(FPh)), for which intramolecular electron transfer is expected to be the fastest among the three binuclear complexes, in the presence of 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH), kET was measured as kET = (1.4 ± 0.1) × 109 s−1. This clearly shows that intramolecular electron transfer in these RuRe binuclear supramolecular photocatalysts is not the rate-determining process in the photocatalytic reduction of CO2, which is one of the main reasons why they work so efficiently.
中文翻译:

使用时间分辨红外测量研究用于 CO2 还原的 Ru(ii)–Re(i) 超分子光催化剂的激发态、还原猝灭和分子内电子转移†
Ru( II )光敏剂和Re( I )催化剂单元通过乙烯连接体相互连接的超分子光催化剂是用于CO 2还原的最著名、最有效和最耐用的光催化系统之一。在本文中,我们首次报告了其中三种双核配合物的时间分辨红外(TRIR)光谱,以揭示催化剂为何如此有效地发挥作用。用 532 nm 激光脉冲选择性激发 Ru 单元,诱导缓慢的分子内电子从Ru 单元的3 MLCT 激发态转移到 Re 单元,主要速率常数为 (1.0–1.1) × 10 4 s -1组分和(3.5–4.3) × 10 6 s -1作为次要组分,在乙腈中。产生的电荷分离态具有很长的寿命,电荷复合速率常数仅为(6.5–8.4) × 10 4 s -1。因此,尽管它具有很大的驱动力(-ΔG 0 CR ∼ 2.6 eV),但该过程处于马库斯反转区域。另一方面,在 1-苄基-1,4-二氢烟酰胺 (BNAH) 存在下,激发的 Ru 单元的还原猝灭进行得更快 ( k q [BNAH (0.2 M)] = (3.5–3.8) × 10 6 s -1 )比上述分子内氧化猝灭,产生Ru单元的单电子还原物质(OERS)。纳秒 TRIR 数据清楚地表明,从 Ru 单元的 OERS 到 Re 单元的分子内电子转移 ( k ET > 2 × 10 7 s -1 ) 比从 Ru 单元的激发态转移要快得多,而且它也是比 BNAH 激发的 Ru 单元的还原猝灭过程更快。为了测量k ET的精确值,使用了皮秒 TRIR 光谱和更强的还原剂。因此,对于具有三(对氟苯基)膦配体(RuRe(FPh))的双核配合物,在存在1,3- 的情况下,分子内电子转移预计是三种双核配合物中最快的。二甲基-2-苯基-2,3-二氢-1 H-苯并[ d ]咪唑(BIH),k ET测量为k ET = (1.4 ± 0.1) × 10 9 s -1。这清楚地表明这些RuRe双核超分子光催化剂中的分子内电子转移并不是光催化还原CO 2的速率决定过程,这是他们工作如此高效的主要原因之一。
更新日期:2018-02-14
中文翻译:

使用时间分辨红外测量研究用于 CO2 还原的 Ru(ii)–Re(i) 超分子光催化剂的激发态、还原猝灭和分子内电子转移†
Ru( II )光敏剂和Re( I )催化剂单元通过乙烯连接体相互连接的超分子光催化剂是用于CO 2还原的最著名、最有效和最耐用的光催化系统之一。在本文中,我们首次报告了其中三种双核配合物的时间分辨红外(TRIR)光谱,以揭示催化剂为何如此有效地发挥作用。用 532 nm 激光脉冲选择性激发 Ru 单元,诱导缓慢的分子内电子从Ru 单元的3 MLCT 激发态转移到 Re 单元,主要速率常数为 (1.0–1.1) × 10 4 s -1组分和(3.5–4.3) × 10 6 s -1作为次要组分,在乙腈中。产生的电荷分离态具有很长的寿命,电荷复合速率常数仅为(6.5–8.4) × 10 4 s -1。因此,尽管它具有很大的驱动力(-ΔG 0 CR ∼ 2.6 eV),但该过程处于马库斯反转区域。另一方面,在 1-苄基-1,4-二氢烟酰胺 (BNAH) 存在下,激发的 Ru 单元的还原猝灭进行得更快 ( k q [BNAH (0.2 M)] = (3.5–3.8) × 10 6 s -1 )比上述分子内氧化猝灭,产生Ru单元的单电子还原物质(OERS)。纳秒 TRIR 数据清楚地表明,从 Ru 单元的 OERS 到 Re 单元的分子内电子转移 ( k ET > 2 × 10 7 s -1 ) 比从 Ru 单元的激发态转移要快得多,而且它也是比 BNAH 激发的 Ru 单元的还原猝灭过程更快。为了测量k ET的精确值,使用了皮秒 TRIR 光谱和更强的还原剂。因此,对于具有三(对氟苯基)膦配体(RuRe(FPh))的双核配合物,在存在1,3- 的情况下,分子内电子转移预计是三种双核配合物中最快的。二甲基-2-苯基-2,3-二氢-1 H-苯并[ d ]咪唑(BIH),k ET测量为k ET = (1.4 ± 0.1) × 10 9 s -1。这清楚地表明这些RuRe双核超分子光催化剂中的分子内电子转移并不是光催化还原CO 2的速率决定过程,这是他们工作如此高效的主要原因之一。



























 京公网安备 11010802027423号
京公网安备 11010802027423号