当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Time-lapse live cell imaging to monitor doxorubicin release from DNA origami nanostructures†
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1039/c7tb03223d Yun Zeng 1, 2, 3, 4 , Jiajun Liu 4, 5, 6, 7, 8 , Shuo Yang 1, 2, 3, 4 , Wenyan Liu 2, 3, 4, 9 , Liang Xu 4, 5, 6, 7, 8 , Risheng Wang 1, 2, 3, 4
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1039/c7tb03223d Yun Zeng 1, 2, 3, 4 , Jiajun Liu 4, 5, 6, 7, 8 , Shuo Yang 1, 2, 3, 4 , Wenyan Liu 2, 3, 4, 9 , Liang Xu 4, 5, 6, 7, 8 , Risheng Wang 1, 2, 3, 4
Affiliation
|
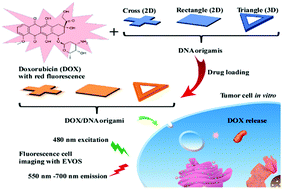
Self-assembled DNA nanostructures have attracted significant research interest in biomedical applications because of their excellent programmability and biocompatibility. To develop multifunctional drug delivery from DNA nanostructures, considerable key information is still needed for clinical application. Traditional fixed endpoint assays do not reflect the dynamic and heterogeneous responses of cells with regard to drugs, and may lead to the misinterpretation of experimental results. For the first time, an integrated time-lapse live cell imaging system was used to study the cellular internalization and controlled drug release profile of three different shaped DNA origami/doxorubicin (DOX) complexes for three days. Our results demonstrated the dependence of DNA nanostructures on shape for drug delivery efficiency, while the rigid 3D DNA origami triangle frame exhibited enhanced cellular uptake capability, as compared with flexible 2D DNA structures. In addition, the translocation of released DOX into the nucleus was proved by fluorescence microscopy, in which a DOX-loaded 3D DNA triangle frame displayed a stronger accumulation of DOX in nuclei. Moreover, given the facile drug loading and auto fluorescence of the anti-cancer drug, DOX, our results suggest that the DNA nanostructure is a promising candidate, as a label-free nanocarrier, for DOX delivery, with great potential for anticancer therapy as well.
中文翻译:

延时活细胞成像以监测阿霉素从DNA折纸纳米结构中释放的情况†
自组装的DNA纳米结构因其出色的可编程性和生物相容性,在生物医学应用中引起了广泛的研究兴趣。为了从DNA纳米结构开发多功能药物递送,临床应用仍需要大量关键信息。传统的固定终点检测无法反映细胞对药物的动态和异质反应,并可能导致对实验结果的误解。首次使用集成的延时活细胞成像系统研究了三种不同形状的DNA折纸/阿霉素(DOX)复合物的细胞内在化和受控药物释放曲线,为期三天。我们的结果证明了DNA纳米结构对形状的依赖性,从而提高了药物的递送效率,与刚性2D DNA结构相比,刚性3D DNA折纸三角形框架显示出增强的细胞摄取能力。此外,通过荧光显微镜证实了释放的DOX易位到核中,其中装载有DOX的3D DNA三角框架显示了DOX在核中的更强积累。此外,考虑到抗癌药物DOX的简便药物装载和自发荧光,我们的研究结果表明,DNA纳米结构作为无标记的纳米载体,有望成为DOX递送的有希望的候选者,并且在抗癌治疗方面也具有巨大潜力。其中装载有DOX的3D DNA三角框架显示了DOX在核中的更强堆积。此外,考虑到抗癌药物DOX的简便药物装载和自发荧光,我们的结果表明,DNA纳米结构作为无标记的纳米载体,有望成为DOX的有前途候选者,并且在抗癌治疗方面也具有巨大潜力。其中装载有DOX的3D DNA三角框架显示了DOX在核中的更强堆积。此外,考虑到抗癌药物DOX的简便药物装载和自发荧光,我们的研究结果表明,DNA纳米结构作为无标记的纳米载体,有望成为DOX递送的有希望的候选者,并且在抗癌治疗方面也具有巨大潜力。
更新日期:2018-02-13
中文翻译:

延时活细胞成像以监测阿霉素从DNA折纸纳米结构中释放的情况†
自组装的DNA纳米结构因其出色的可编程性和生物相容性,在生物医学应用中引起了广泛的研究兴趣。为了从DNA纳米结构开发多功能药物递送,临床应用仍需要大量关键信息。传统的固定终点检测无法反映细胞对药物的动态和异质反应,并可能导致对实验结果的误解。首次使用集成的延时活细胞成像系统研究了三种不同形状的DNA折纸/阿霉素(DOX)复合物的细胞内在化和受控药物释放曲线,为期三天。我们的结果证明了DNA纳米结构对形状的依赖性,从而提高了药物的递送效率,与刚性2D DNA结构相比,刚性3D DNA折纸三角形框架显示出增强的细胞摄取能力。此外,通过荧光显微镜证实了释放的DOX易位到核中,其中装载有DOX的3D DNA三角框架显示了DOX在核中的更强积累。此外,考虑到抗癌药物DOX的简便药物装载和自发荧光,我们的研究结果表明,DNA纳米结构作为无标记的纳米载体,有望成为DOX递送的有希望的候选者,并且在抗癌治疗方面也具有巨大潜力。其中装载有DOX的3D DNA三角框架显示了DOX在核中的更强堆积。此外,考虑到抗癌药物DOX的简便药物装载和自发荧光,我们的结果表明,DNA纳米结构作为无标记的纳米载体,有望成为DOX的有前途候选者,并且在抗癌治疗方面也具有巨大潜力。其中装载有DOX的3D DNA三角框架显示了DOX在核中的更强堆积。此外,考虑到抗癌药物DOX的简便药物装载和自发荧光,我们的研究结果表明,DNA纳米结构作为无标记的纳米载体,有望成为DOX递送的有希望的候选者,并且在抗癌治疗方面也具有巨大潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号