当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A solvent-free, base-catalyzed domino reaction towards trifluoromethylated benzenes from bio-based methyl coumalate
Green Chemistry ( IF 9.8 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1039/c7gc03721j Liang Chang 1, 2, 3, 4, 5 , Nadja Klipfel 1, 2, 3, 4, 5 , Luc Dechoux 1, 2, 3, 4, 5 , Serge Thorimbert 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1039/c7gc03721j Liang Chang 1, 2, 3, 4, 5 , Nadja Klipfel 1, 2, 3, 4, 5 , Luc Dechoux 1, 2, 3, 4, 5 , Serge Thorimbert 1, 2, 3, 4, 5
Affiliation
|
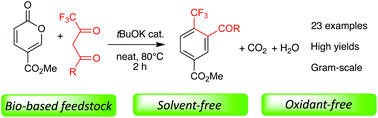
A novel, efficient, and environmentally compatible method for CF3-substituted benzene production is reported. It sources a bio-based feedstock, employs tBuOK as a catalyst, and is solvent-free. This regioselective approach provides various trifluoromethyl benzenes in good to excellent yields, without any extra oxidant or special care. CO2 and water are the only byproducts of this process, and the reaction conditions can scale up to gram quantities. The transformation involves an unprecedented tBuOK-catalyzed domino process, and features Michael addition/6π-electrocyclic ring opening/[1,5]-H shift/carba-6π-electrocyclic ring closure/decarboxylative aromatization reactions.
中文翻译:

从生物基香豆酸甲酯向三氟甲基化苯的无溶剂碱催化多米诺反应
报道了一种用于CF 3取代的苯生产的新颖,有效且与环境相容的方法。它以生物基原料为原料,使用t BuOK作为催化剂,并且不含溶剂。这种区域选择性的方法可以很好地提供各种三氟甲基苯,而无需任何额外的氧化剂或特别的照顾。CO 2和水是该过程的唯一副产物,反应条件可以扩展到克量。该转化涉及前所未有的t BuOK催化的多米诺骨牌过程,并具有迈克尔加成/6π-电环开环/ [1,5] -H移位/carba-6π-电环闭环/脱羧芳构化反应。
更新日期:2018-04-03
中文翻译:

从生物基香豆酸甲酯向三氟甲基化苯的无溶剂碱催化多米诺反应
报道了一种用于CF 3取代的苯生产的新颖,有效且与环境相容的方法。它以生物基原料为原料,使用t BuOK作为催化剂,并且不含溶剂。这种区域选择性的方法可以很好地提供各种三氟甲基苯,而无需任何额外的氧化剂或特别的照顾。CO 2和水是该过程的唯一副产物,反应条件可以扩展到克量。该转化涉及前所未有的t BuOK催化的多米诺骨牌过程,并具有迈克尔加成/6π-电环开环/ [1,5] -H移位/carba-6π-电环闭环/脱羧芳构化反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号