当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Glycoconjugate Oxime Formation Catalyzed at Neutral pH: Mechanistic Insights and Applications of 1,4-Diaminobenzene as a Superior Catalyst for Complex Carbohydrates
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00019 Mads Østergaard 1 , Niels Johan Christensen 1 , Christian T. Hjuler 1 , Knud J. Jensen 1 , Mikkel B. Thygesen 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00019 Mads Østergaard 1 , Niels Johan Christensen 1 , Christian T. Hjuler 1 , Knud J. Jensen 1 , Mikkel B. Thygesen 1
Affiliation
|
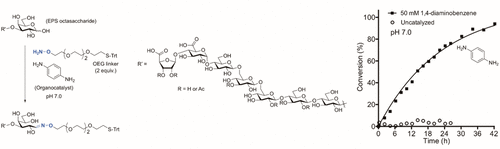
The reaction of unprotected carbohydrates with aminooxy reagents to provide oximes is a key method for the construction of glycoconjugates. Aniline and derivatives serve as organocatalysts for the formation of oximes from simple aldehydes, and we have previously reported that aniline also catalyzes the formation of oximes from the more complex aldehydes, carbohydrates. Here, we present a comprehensive study of the effect of aniline analogues on the formation of carbohydrate oximes and related glycoconjugates depending on organocatalyst structure, pH, nucleophile, and carbohydrate, covering more than 150 different reaction conditions. The observed superiority of the 1,4-diaminobenzene (PDA) catalyst at neutral pH is rationalized by NMR analyses and DFT studies of reaction intermediates. Carbohydrate oxime formation at pH 7 is demonstrated by the formation of a bioactive glycoconjugate from a labile, decorated octasaccharide originating from exopolysaccharides of the soil bacterium Mesorhizobium loti. This study of glycoconjugate formation includes the first direct comparison of aniline-catalyzed reaction rates and equilibrium constants for different classes of nucleophiles, including primary oxyamines, secondary N-alkyl oxyamines, as well as aryl and arylsulfonyl hydrazides. We identified 1,4-diaminobenzene as a superior catalyst for the construction of oxime-linked glycoconjugates under mild conditions.
中文翻译:

在中性pH下催化糖共轭肟的形成:机理研究和1,4-二氨基苯作为复杂碳水化合物的高级催化剂的应用
未保护的碳水化合物与氨氧基试剂反应生成肟是构建糖缀合物的关键方法。苯胺和衍生物可作为由简单醛形成肟的有机催化剂,并且我们以前曾报道苯胺还催化由较复杂的醛(碳水化合物)形成肟。在这里,我们根据有机催化剂的结构,pH,亲核试剂和碳水化合物对苯胺类似物对碳水化合物肟和相关糖缀合物形成的影响进行了全面研究,涵盖了150多种不同的反应条件。通过NMR分析和反应中间体的DFT研究,可以合理地观察到1,4-二氨基苯(PDA)催化剂在中性pH下的优越性。中型根瘤菌。糖缀合物形成的这项研究包括首次直接比较苯胺催化的反应速率和不同类别亲核试剂(包括伯氧胺,仲N烷基氧胺以及芳基和芳基磺酰肼)的平衡常数。我们确定1,4-二氨基苯是在温和条件下构建肟连接的糖缀合物的优良催化剂。
更新日期:2018-02-13
中文翻译:

在中性pH下催化糖共轭肟的形成:机理研究和1,4-二氨基苯作为复杂碳水化合物的高级催化剂的应用
未保护的碳水化合物与氨氧基试剂反应生成肟是构建糖缀合物的关键方法。苯胺和衍生物可作为由简单醛形成肟的有机催化剂,并且我们以前曾报道苯胺还催化由较复杂的醛(碳水化合物)形成肟。在这里,我们根据有机催化剂的结构,pH,亲核试剂和碳水化合物对苯胺类似物对碳水化合物肟和相关糖缀合物形成的影响进行了全面研究,涵盖了150多种不同的反应条件。通过NMR分析和反应中间体的DFT研究,可以合理地观察到1,4-二氨基苯(PDA)催化剂在中性pH下的优越性。中型根瘤菌。糖缀合物形成的这项研究包括首次直接比较苯胺催化的反应速率和不同类别亲核试剂(包括伯氧胺,仲N烷基氧胺以及芳基和芳基磺酰肼)的平衡常数。我们确定1,4-二氨基苯是在温和条件下构建肟连接的糖缀合物的优良催化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号