Molecular Therapy - Nucleic Acids ( IF 8.8 ) Pub Date : 2018-02-13 , DOI: 10.1016/j.omtn.2018.02.003 Anne Marit de Groot 1 , Kaushik Thanki 2 , Monique Gangloff 3 , Emily Falkenberg 2 , Xianghui Zeng 2 , Djai C J van Bijnen 1 , Willem van Eden 1 , Henrik Franzyk 4 , Hanne M Nielsen 2 , Femke Broere 1 , Nick J Gay 3 , Camilla Foged 2 , Alice J A M Sijts 1
|
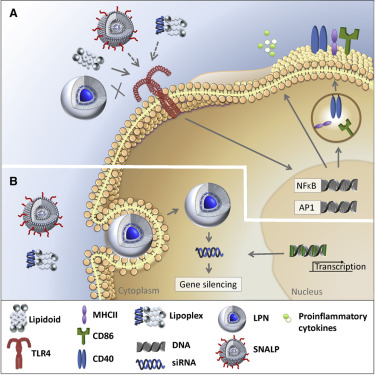
Therapeutics based on small interfering RNA (siRNA) have promising potential as antiviral and anti-inflammatory agents. To deliver siRNA across cell membranes to reach the RNAi pathway in the cytosol of target cells, non-viral nanoparticulate delivery approaches are explored. Recently, we showed that encapsulation of siRNA in lipid-polymer hybrid nanoparticles (LPNs), based on poly(DL-lactic-co-glycolic acid) (PLGA) and cationic lipid-like materials (lipidoids), remarkably enhances intracellular delivery of siRNA as compared to siRNA delivery with LPNs modified with dioleoyltrimethylammoniumpropane (DOTAP) as the lipid component. However, the potential immune modulation by these cationic lipids remains unexplored. By testing lipidoids and DOTAP for innate immune-receptor-activating properties in vitro, we found that neither lipidoids nor DOTAP activate human Toll-like receptor (TLR) 2, 3, 7, and 9. However, in contrast to DOTAP, lipidoids are strong agonists for TLR4 and activate murine antigen-presenting cells in vitro. This agonistic effect was further confirmed in silico using a prediction model based on crystal structures. Also, lipidoids formulated as lipoplexes or as stable nucleic acid lipid particles, which was the reference formulation for siRNA delivery, proved to activate TLR4. However, by combining lipidoids with PLGA into LPNs, TLR4 activation was abrogated. Thus, lipidoid-mediated TLR4 activation during siRNA delivery may be modulated via optimization of the formulation design.
中文翻译:

脂质体内和体外的免疫原性测试:通过纳米颗粒设计调节脂质介导的TLR4活化。
基于小干扰RNA(siRNA)的治疗剂作为抗病毒和抗炎药具有广阔的前景。为了跨细胞膜传递siRNA到达靶细胞胞质溶胶中的RNAi途径,人们探索了非病毒纳米颗粒的传递方法。最近,我们发现基于聚(DL-乳酸-乙醇酸)(PLGA)和阳离子脂质样材料(类脂质)的脂质-聚合物杂化纳米颗粒(LPNs)中的siRNA封装显着增强了siRNA的细胞内递送。与siRNA递送相比,其中LPNs修饰为油酰基三甲基铵丙烷(DOTAP)作为脂质成分。然而,这些阳离子脂质潜在的免疫调节仍未探索。通过测试类脂质和DOTAP先天免疫受体活化性能的体外,我们发现类脂质和DOTAP均未激活人Toll样受体(TLR)2、3、7和9。但是,与DOTAP相比,类脂质是TLR4的强激动剂,并在体外激活鼠类抗原呈递细胞。使用基于晶体结构的预测模型在计算机上进一步证实了这种激动作用。同样,被证明是脂质复合物或稳定的核酸脂质颗粒的类脂质,是siRNA递送的参考制剂,被证明可以激活TLR4。但是,通过将类脂质和PLGA组合成LPN,可以消除TLR4激活。因此,可以通过优化制剂设计来调节在siRNA递送期间类脂质介导的TLR4活化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号