当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Situ Target Engagement Studies in Adherent Cells
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-02-12 00:00:00 , DOI: 10.1021/acschembio.7b01079 Hanna Axelsson 1, 2 , Helena Almqvist 1, 2 , Magdalena Otrocka 1, 2 , Michaela Vallin 1, 2 , Sara Lundqvist 3 , Pia Hansson 3 , Ulla Karlsson 3 , Thomas Lundbäck 1, 2, 3 , Brinton Seashore-Ludlow 1, 4
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-02-12 00:00:00 , DOI: 10.1021/acschembio.7b01079 Hanna Axelsson 1, 2 , Helena Almqvist 1, 2 , Magdalena Otrocka 1, 2 , Michaela Vallin 1, 2 , Sara Lundqvist 3 , Pia Hansson 3 , Ulla Karlsson 3 , Thomas Lundbäck 1, 2, 3 , Brinton Seashore-Ludlow 1, 4
Affiliation
|
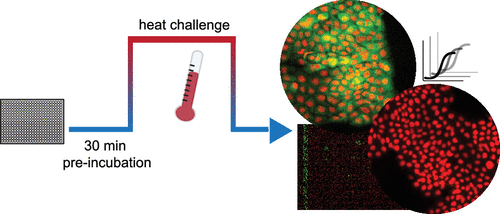
A prerequisite for successful drugs is effective binding of the desired target protein in the complex environment of a living system. Drug–target engagement has typically been difficult to monitor in physiologically relevant models, and with current methods, especially, while maintaining spatial information. One recent technique for quantifying drug–target engagement is the cellular thermal shift assay (CETSA), in which ligand-induced protein stabilization is measured after a heat challenge. Here, we describe a CETSA protocol in live A431 cells for p38α (MAPK14), where remaining soluble protein is detected in situ, using high-content imaging in 384-well, microtiter plates. We validate this assay concept using a number of known p38α inhibitors and further demonstrate the potential of this technology for chemical probe and drug discovery purposes by performing a small pilot screen for novel p38α binders. Importantly, this protocol creates a workflow that is amenable to adherent cells in their native state and yields spatially resolved target engagement information measurable at the single-cell level.
中文翻译:

贴壁细胞的原位靶标参与研究
成功的药物的先决条件是在复杂的生命系统环境中有效结合所需的目标蛋白。药物-靶标参与通常很难在生理相关模型中进行监测,尤其是在保持空间信息的情况下,难以通过当前方法进行监测。一种用于量化药物与靶标结合的最新技术是细胞热位移分析(CETSA),其中在热刺激后测量配体诱导的蛋白质稳定性。在这里,我们描述了在活的A431细胞中针对p38α(MAPK14)的CETSA方案,其中原位检测到了剩余的可溶性蛋白在384孔微量滴定板中使用高内涵成像。我们使用许多已知的p38α抑制剂验证了这一测定概念,并通过对新型p38α结合剂进行了小型中试筛选,进一步证明了该技术在化学探针和药物发现方面的潜力。重要的是,该协议创建了一个工作流,该工作流适合于处于原始状态的贴壁细胞,并产生可在单细胞水平上测量的空间分辨目标参与信息。
更新日期:2018-02-12
中文翻译:

贴壁细胞的原位靶标参与研究
成功的药物的先决条件是在复杂的生命系统环境中有效结合所需的目标蛋白。药物-靶标参与通常很难在生理相关模型中进行监测,尤其是在保持空间信息的情况下,难以通过当前方法进行监测。一种用于量化药物与靶标结合的最新技术是细胞热位移分析(CETSA),其中在热刺激后测量配体诱导的蛋白质稳定性。在这里,我们描述了在活的A431细胞中针对p38α(MAPK14)的CETSA方案,其中原位检测到了剩余的可溶性蛋白在384孔微量滴定板中使用高内涵成像。我们使用许多已知的p38α抑制剂验证了这一测定概念,并通过对新型p38α结合剂进行了小型中试筛选,进一步证明了该技术在化学探针和药物发现方面的潜力。重要的是,该协议创建了一个工作流,该工作流适合于处于原始状态的贴壁细胞,并产生可在单细胞水平上测量的空间分辨目标参与信息。



























 京公网安备 11010802027423号
京公网安备 11010802027423号