当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
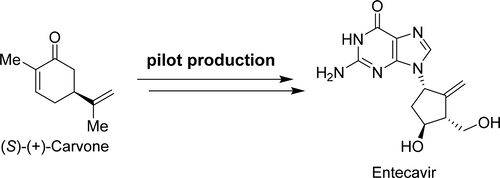
Total Synthesis of Entecavir: A Robust Route for Pilot Production
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-02-12 00:00:00 , DOI: 10.1021/acs.oprd.8b00007 Hua Xu 1 , Fang Wang 1 , Weicai Xue 1 , Yunjie Zheng 1 , Qi Wang 1 , Fayang G. Qiu 1 , Yehua Jin 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-02-12 00:00:00 , DOI: 10.1021/acs.oprd.8b00007 Hua Xu 1 , Fang Wang 1 , Weicai Xue 1 , Yunjie Zheng 1 , Qi Wang 1 , Fayang G. Qiu 1 , Yehua Jin 1
Affiliation
|
A practical synthetic route for pilot production of entecavir is described. It is safe, robust, and scalable to kilogram scale. Starting from (S)-(+)-carvone, this synthetic route consists of a series of highly efficient reactions including a Favorskii rearrangement-elimination-epimerization sequence to establish the cyclopentene skeleton, the Baeyer–Villiger oxidation/rearrangement to afford the correct configuration of the secondary alcohol, and a directed homoallylic epoxidation followed by epoxide ring-opening to introduce the hydroxyl group suitable for the Mitsunobu reaction. In addition, the synthesis contains only four brief chromatographic purifications.
中文翻译:

恩替卡韦的全合成:中试生产的可靠途径
描述了用于恩替卡韦中试生产的实用合成途径。它是安全,可靠且可扩展至公斤级的。从(S)-(+)-香芹酮开始,该合成路线由一系列高效反应组成,包括Favorskii重排-消除-上位化序列以建立环戊烯骨架,Baeyer-Villiger氧化/重排以提供正确的构型然后,进行仲醇的直接芳族环氧化,然后进行环氧化物开环,以引入适合于Mitsunobu反应的羟基。此外,该合成仅包含四个简短的色谱纯化。
更新日期:2018-02-12
中文翻译:

恩替卡韦的全合成:中试生产的可靠途径
描述了用于恩替卡韦中试生产的实用合成途径。它是安全,可靠且可扩展至公斤级的。从(S)-(+)-香芹酮开始,该合成路线由一系列高效反应组成,包括Favorskii重排-消除-上位化序列以建立环戊烯骨架,Baeyer-Villiger氧化/重排以提供正确的构型然后,进行仲醇的直接芳族环氧化,然后进行环氧化物开环,以引入适合于Mitsunobu反应的羟基。此外,该合成仅包含四个简短的色谱纯化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号