Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2018-02-07 , DOI: 10.1016/j.jfluchem.2018.02.005 Huiqin Yin , Peng Zhang , Xuehui An , Jinhui Cheng , Xiang Li , Shuang Wu , Xijun Wu , Wenguan Liu , Leidong Xie
|
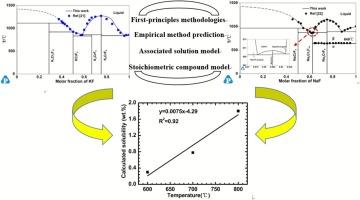
The thermodynamic evaluation and optimizations of the KF-CrF3 and NaF-CrF3 systems were carried out within the framework of CALPHAD (CALculation of PHAse of Diagrams) approach. The liquid phase was described by the associated solution model, and the intermediate phase were treated as stoichiometric compound model. All the model parameters were optimized based on the experimental phase equilibria data from experimental measurements and theoretical predictions (First-principles calculation and empirical equation). A set of self-consistent and reliable thermodynamic parameters was obtained, which can well describe the phase equilibria and thermodynamic properties of the KF-CrF3 and NaF-CrF3 system. Furthermore, the database for the LiF-NaF-KF-CrF3 quaternary system was preliminarily established using Muggianu extrapolation model. Meanwhile, the solubility of CrF3 in FLiNaK at different temperatures was obtained. The calculated value is in good agreement with the reported measurements by ORNL, considering kinetic factor of K3CrF6 involved in FLiNaK.
中文翻译:

LiF-NaF-KF-CrF 3系统的热力学建模
KF-CrF 3和NaF-CrF 3系统的热力学评估和优化是在CALPHAD(图的PHAse的计算)方法的框架内进行的。用相关的溶液模型描述液相,将中间相作为化学计量的化合物模型处理。所有模型参数均基于来自实验测量和理论预测(第一原理计算和经验方程式)的实验相平衡数据进行了优化。获得了一组自洽和可靠的热力学参数,可以很好地描述KF-CrF 3和NaF-CrF 3的相平衡和热力学性质。系统。此外,使用Muggianu外推模型初步建立了LiF-NaF-KF-CrF 3四元体系的数据库。同时,获得了CrF 3在不同温度下在FLiNaK中的溶解度。考虑到参与FLiNaK的K 3 CrF 6的动力学因子,计算值与ORNL报告的测量值非常吻合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号