Electrochemistry Communications ( IF 5.4 ) Pub Date : 2018-02-08 , DOI: 10.1016/j.elecom.2018.02.005 Corie Horwood , Michael Stadermann
|
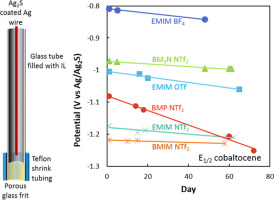
We report on a reference electrode designed for use in ionic liquids, based on a silver wire coated with silver sulfide. The reference electrode potential is determined by the concentrations of Ag+ and S2−, which are established by the solubility of the Ag2S coating on the Ag wire. While potential shifts of >100 mV during an experiment have been reported when using silver or platinum wire quasi-reference electrodes, the reference electrode reported here provides a stable potential over several months of experimental use. Additionally, our reference electrode can be prepared and used in a normal air atmosphere, and does not need to be assembled and used in a glovebox, or protected from light. The reference electrode has been characterized by voltammetry measurements of ferrocene and cobaltocenium hexafluorophosphate, and was found to slowly drift to more positive potentials at a rate of <1 mV/day for five of the six ionic liquids investigated.
中文翻译:

对离子液体中电化学具有长期稳定性的Ag / Ag 2 S参比电极的评估
我们报道了一种设计用于离子液体的参比电极,该参比电极基于涂有硫化银的银线。参比电极的电势由Ag +和S 2-的浓度确定,这由Ag 2的溶解度确定银丝上的S涂层。虽然在使用银或铂丝准参比电极时已报告了实验过程中> 100 mV的电位偏移,但此处报告的参比电极在几个月的实验使用中提供了稳定的电位。此外,我们的参比电极可以在正常的大气环境中制备和使用,不需要在手套箱中组装和使用,也可以避光。参比电极已通过二茂铁和六氟磷酸钴钴的伏安法测量进行了表征,并且发现对于所研究的六种离子液体中的五种,其以<1 mV /天的速率缓慢漂移至更高的正电势。



























 京公网安备 11010802027423号
京公网安备 11010802027423号