当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
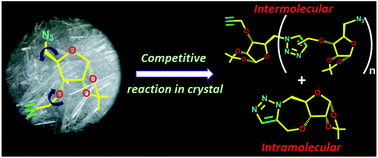
Three-way competition in a topochemical reaction: permutative azide–alkyne cycloaddition reactions leading to a vast library of products in the crystal†
CrystEngComm ( IF 3.1 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1039/c8ce00131f Kuntrapakam Hema 1, 2, 3 , Kana M. Sureshan 1, 2, 3
CrystEngComm ( IF 3.1 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1039/c8ce00131f Kuntrapakam Hema 1, 2, 3 , Kana M. Sureshan 1, 2, 3
Affiliation
|
5-Azido-3-O-propargyl-1,2-O-isopropylidene-α-D-ribofuranose (1) showed the possibility of undergoing topochemical azide–alkyne cycloaddition (TAAC) reaction to form 1,5-triazolyl linked polymers in the crystal. Surprisingly, when thermally activated, the molecules reach a conformational equilibrium wherein the native conformer reacts intermolecularly to form a 1,5-triazolyl linkage but the other conformer reacts either intermolecularly to form a 1,4-triazolyl linkage or intramolecularly to form 2. Thus, the TAAC reaction adopts three competing pathways, of which two generate two different types of linkages for chain-growth and the third one generates a chain breaker. While the random occurrence of the chain-breaker reaction ensures the formation of oligomers of different sizes, the permutative occurrence of two types of linkages in any n-mer produces 2n different oligomers of the same size.
中文翻译:

拓扑化学反应中的三向竞争:叠氮化物-炔烃环加成反应导致晶体中大量产物的形成†
5-叠氮基-3- O-炔丙基-1,2- O-异亚丙基-α- D-呋喃核糖(1)显示可能发生拓扑化学叠氮化物-炔烃环加成(TAAC)反应形成1,5-三唑基连接的聚合物水晶。令人惊讶地,当被热活化时,分子达到构象平衡,其中天然构象异构体在分子间反应形成1,5-三唑基键,而另一个构象异构体在分子间反应形成1,4-三唑基键或在分子内反应形成2。。因此,TAAC反应采用三个竞争途径,其中两个产生链增长的两种不同类型的连接,第三个产生链断裂剂。尽管随机发生的链断裂反应可确保形成不同大小的低聚物,但在任何n -mer中两种键的排列发生均会产生2 n个相同大小的不同低聚物。
更新日期:2018-02-07
中文翻译:

拓扑化学反应中的三向竞争:叠氮化物-炔烃环加成反应导致晶体中大量产物的形成†
5-叠氮基-3- O-炔丙基-1,2- O-异亚丙基-α- D-呋喃核糖(1)显示可能发生拓扑化学叠氮化物-炔烃环加成(TAAC)反应形成1,5-三唑基连接的聚合物水晶。令人惊讶地,当被热活化时,分子达到构象平衡,其中天然构象异构体在分子间反应形成1,5-三唑基键,而另一个构象异构体在分子间反应形成1,4-三唑基键或在分子内反应形成2。。因此,TAAC反应采用三个竞争途径,其中两个产生链增长的两种不同类型的连接,第三个产生链断裂剂。尽管随机发生的链断裂反应可确保形成不同大小的低聚物,但在任何n -mer中两种键的排列发生均会产生2 n个相同大小的不同低聚物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号