当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tuning protein assembly pathways through superfast amyloid-like aggregation†
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1039/c8bm00066b Chen Li 1, 2, 3, 4, 5 , Lu Xu 6, 7, 8, 9 , Yi Y. Zuo 6, 7, 8, 9 , Peng Yang 1, 2, 3, 4, 5
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1039/c8bm00066b Chen Li 1, 2, 3, 4, 5 , Lu Xu 6, 7, 8, 9 , Yi Y. Zuo 6, 7, 8, 9 , Peng Yang 1, 2, 3, 4, 5
Affiliation
|
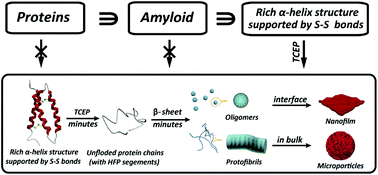
Amyloid formation of proteins is not only relevant for neurodegenerative diseases, but has recently emerged as a groundbreaking approach in materials science and biotechnology. However, amyloid aggregation of proteins in vitro generally requires a long incubation time under extremely harsh conditions, and the understanding of the structural motif to determine amyloid assembly is extremely limited. Herein we reveal that the integration of three important building blocks in typical globular proteins is crucial for superfast protein amyloid-like assembly including the segment required for high fibrillation propensity, abundant α-helix structures and intramolecular S–S bonds to lock the α-helix. With the reduction of the S–S bond by tris(2-carboxyethyl)phosphine (TCEP), the α-helix was rapidly unlocked from the protein chain, and the resultant unfolded monomer underwent a fast transition to β-sheet-rich amyloid oligomers and protofibrils in minutes, which further assembled into a macroscopic nanofilm at the air/water interface and microparticles in bulk solution, respectively.
中文翻译:

通过超快淀粉样样聚集来调节蛋白质组装途径†
蛋白质的淀粉样蛋白形成不仅与神经退行性疾病有关,而且最近在材料科学和生物技术中已经成为一种突破性的方法。但是,淀粉样蛋白在体外的聚集通常在极端苛刻的条件下需要较长的孵育时间,并且对确定淀粉样蛋白组装体的结构基序的理解极为有限。本文揭示了典型球状蛋白质中三个重要组成部分的整合对于超快蛋白质淀粉样蛋白组装至关重要,包括高原纤维化倾向,丰富的α-螺旋结构和分子内S–S键锁定α-螺旋所需的片段。随着三(2-羧乙基)膦(TCEP)还原S–S键,α-螺旋迅速从蛋白质链中解开,并且所得的未折叠单体经历了向富含β-折叠的淀粉样蛋白低聚物的快速转变。和原纤维在几分钟之内,然后在空气/水界面处进一步组装成宏观纳米薄膜,并在整体溶液中组装成微粒,
更新日期:2018-02-07
中文翻译:

通过超快淀粉样样聚集来调节蛋白质组装途径†
蛋白质的淀粉样蛋白形成不仅与神经退行性疾病有关,而且最近在材料科学和生物技术中已经成为一种突破性的方法。但是,淀粉样蛋白在体外的聚集通常在极端苛刻的条件下需要较长的孵育时间,并且对确定淀粉样蛋白组装体的结构基序的理解极为有限。本文揭示了典型球状蛋白质中三个重要组成部分的整合对于超快蛋白质淀粉样蛋白组装至关重要,包括高原纤维化倾向,丰富的α-螺旋结构和分子内S–S键锁定α-螺旋所需的片段。随着三(2-羧乙基)膦(TCEP)还原S–S键,α-螺旋迅速从蛋白质链中解开,并且所得的未折叠单体经历了向富含β-折叠的淀粉样蛋白低聚物的快速转变。和原纤维在几分钟之内,然后在空气/水界面处进一步组装成宏观纳米薄膜,并在整体溶液中组装成微粒,



























 京公网安备 11010802027423号
京公网安备 11010802027423号