当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
LonB Protease Is a Novel Regulator of Carotenogenesis Controlling Degradation of Phytoene Synthase in Haloferax volcanii
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2018-02-15 00:00:00 , DOI: 10.1021/acs.jproteome.7b00809 Micaela Cerletti 1 , Roberto Paggi 1 , Christian Troetschel 2 , María Celeste Ferrari 1 , Carina Ramallo Guevara 2 , Stefan Albaum 3 , Ansgar Poetsch 2, 4 , Rosana De Castro 1
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2018-02-15 00:00:00 , DOI: 10.1021/acs.jproteome.7b00809 Micaela Cerletti 1 , Roberto Paggi 1 , Christian Troetschel 2 , María Celeste Ferrari 1 , Carina Ramallo Guevara 2 , Stefan Albaum 3 , Ansgar Poetsch 2, 4 , Rosana De Castro 1
Affiliation
|
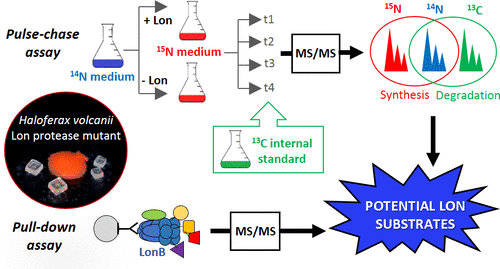
The membrane protease LonB is an essential protein in the archaeon Haloferax volcanii and globally impacts its physiology. However, natural substrates of the archaeal Lon protease have not been identified. The whole proteome turnover was examined in a H. volcanii LonB mutant under reduced and physiological protease levels. LC–MS/MS combined with stable isotope labeling was applied for the identification/quantitation of membrane and cytoplasm proteins. Differential synthesis and degradation rates were evidenced for 414 proteins in response to Lon expression. A total of 58 proteins involved in diverse cellular processes showed a degradation pattern (none/very little degradation in the absence of Lon and increased degradation in the presence of Lon) consistent with a LonB substrate, which was further substantiated for several of these candidates by pull-down assays. The most notable was phytoene synthase (PSY), the rate-limiting enzyme in carotenoid biosynthesis. The rapid degradation of PSY upon LonB induction in addition to the remarkable stabilization of this protein and hyperpigmentation phenotype in the Lon mutant strongly suggest that PSY is a LonB substrate. This work identifies for the first time candidate targets of the archaeal Lon protease and establishes proteolysis by Lon as a novel post-translational regulatory mechanism of carotenogenesis.
中文翻译:

LonB蛋白酶是在八氢番茄红素合成酶的胡萝卜素控制的新的调节降解富饶
膜蛋白酶LonB是古细菌Haloferax volcanii中的必需蛋白,并在全球范围内影响其生理功能。然而,尚未鉴定古细菌Lon蛋白酶的天然底物。在火山嗜血杆菌中检查了整个蛋白质组的周转率LonB突变体在降低的和生理的蛋白酶水平下。LC-MS / MS与稳定同位素标记相结合用于膜和细胞质蛋白的鉴定/定量。证明414种蛋白质响应Lon表达的差异合成和降解速率。共有58种蛋白质参与了不同的细胞过程,显示出与LonB底物一致的降解模式(在没有Lon的情况下几乎没有/几乎没有降解,在Lon的存在下几乎没有降解),这对于其中的几种候选物进一步得到了证实。下拉测定法。最值得注意的是八氢番茄红素合酶(PSY),它是类胡萝卜素生物合成中的限速酶。LonB诱导后,PSY的快速降解以及该蛋白的显着稳定以及Lon突变体中的色素沉着表型强烈表明,PSY是LonB底物。这项工作首次确定了古细菌Lon蛋白酶的候选靶标,并建立了Lon的蛋白水解作用,将其作为新的类胡萝卜素翻译后调控机制。
更新日期:2018-02-16
中文翻译:

LonB蛋白酶是在八氢番茄红素合成酶的胡萝卜素控制的新的调节降解富饶
膜蛋白酶LonB是古细菌Haloferax volcanii中的必需蛋白,并在全球范围内影响其生理功能。然而,尚未鉴定古细菌Lon蛋白酶的天然底物。在火山嗜血杆菌中检查了整个蛋白质组的周转率LonB突变体在降低的和生理的蛋白酶水平下。LC-MS / MS与稳定同位素标记相结合用于膜和细胞质蛋白的鉴定/定量。证明414种蛋白质响应Lon表达的差异合成和降解速率。共有58种蛋白质参与了不同的细胞过程,显示出与LonB底物一致的降解模式(在没有Lon的情况下几乎没有/几乎没有降解,在Lon的存在下几乎没有降解),这对于其中的几种候选物进一步得到了证实。下拉测定法。最值得注意的是八氢番茄红素合酶(PSY),它是类胡萝卜素生物合成中的限速酶。LonB诱导后,PSY的快速降解以及该蛋白的显着稳定以及Lon突变体中的色素沉着表型强烈表明,PSY是LonB底物。这项工作首次确定了古细菌Lon蛋白酶的候选靶标,并建立了Lon的蛋白水解作用,将其作为新的类胡萝卜素翻译后调控机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号