Joule ( IF 39.8 ) Pub Date : 2018-02-06 , DOI: 10.1016/j.joule.2018.01.008 Bryan M. Hunter , Niklas B. Thompson , Astrid M. Müller , George R. Rossman , Michael G. Hill , Jay R. Winkler , Harry B. Gray
|
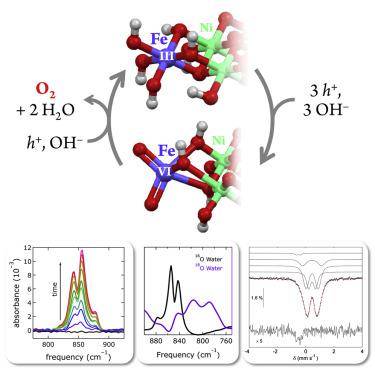
We report in situ spectroscopic measurements in nonaqueous media designed to trap an exceptionally strong oxidant generated electrochemically from an iron-containing nickel layered double hydroxide ([NiFe]-LDH) material. Anodic polarization of this material in acetonitrile produces metal-oxo vibrational spectroscopic signatures along with an extremely narrow near-infrared luminescence peak that strongly indicate that the reactive intermediate is cis-dioxo-iron(VI). Chemical trapping experiments reveal that addition of H2O to the polarized electrochemical cell produces hydrogen peroxide; and, most importantly, addition of HO– generates oxygen. Repolarization of the electrode restores the iron(VI) spectroscopic features, confirming that the high-valent oxo complex is active in the electrocatalytic water oxidation cycle.
中文翻译:

在非水介质中捕集铁(VI)分解水的中间体
我们报告了在非水介质中的原位光谱测量结果,该介质旨在捕获由含铁的镍层状双氢氧化物([NiFe] -LDH)材料电化学生成的异常强的氧化剂。这种材料在乙腈中的阳极极化会产生金属-氧代振动光谱信号,以及一个非常窄的近红外发光峰,这强烈表明该反应性中间体是 顺式-二氧代-铁(VI)。化学捕集实验表明,向极化电化学电池中添加H 2 O会产生过氧化氢。最重要的是,添加HO –产生氧气。电极的重新极化可恢复铁(VI)的光谱特征,从而证实高价氧合络合物在电催化水氧化循环中具有活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号