当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The reaction mechanism and selectivity of acetylene hydrogenation over Ni–Ga intermetallic compound catalysts: a density functional theory study†
Dalton Transactions ( IF 4 ) Pub Date : 2018-02-06 00:00:00 , DOI: 10.1039/c7dt04726f De-Ming Rao 1, 2, 3, 4 , Shi-Tong Zhang 1, 2, 3, 4 , Chang-Ming Li 1, 2, 3, 4 , Yu-Di Chen 4, 5, 6 , Min Pu 1, 2, 3, 4 , Hong Yan 1, 2, 3, 4 , Min Wei 1, 2, 3, 4
Dalton Transactions ( IF 4 ) Pub Date : 2018-02-06 00:00:00 , DOI: 10.1039/c7dt04726f De-Ming Rao 1, 2, 3, 4 , Shi-Tong Zhang 1, 2, 3, 4 , Chang-Ming Li 1, 2, 3, 4 , Yu-Di Chen 4, 5, 6 , Min Pu 1, 2, 3, 4 , Hong Yan 1, 2, 3, 4 , Min Wei 1, 2, 3, 4
Affiliation
|
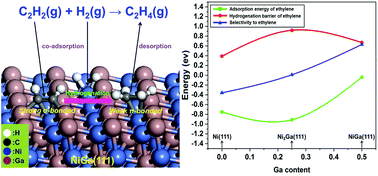
Intermetallic compounds (IMCs) have shown excellent catalytic performance toward the selective hydrogenation of acetylene, but the theoretical understanding on this reaction over Ni-based IMCs is rather limited. In this work, the adsorptions of the C2 species, Bader charge, projected density of states (PDOS) and the reaction pathways were calculated by the density functional theory (DFT) method to investigate the mechanism and selectivity for the acetylene hydrogenation on the (111) surface of NinGa (n = 1, 3) IMCs, with a comparative study on the pristine Ni(111) surface. The results indicate that the adsorption energy of acetylene increased along with the Ni/Ga ratio, therefore a feasible acetylene adsorption on the Ga-rich surface guaranteed a low effective barrier, leading to the best activity for the NiGa(111) surface among three surfaces. Bader charge analysis shows that electrons transferred from Ga atoms to Ni atoms and further delivered to C2 species, decreasing the adsorption capacity of C2 species on NiGa(111) in comparison with those on Ni(111) and Ni3Ga(111). The reaction pathway of acetylene hydrogenation to ethylene via vinyl or even over-hydrogenation to ethane via ethyl is more favorable than the pathway involving the ethylidene intermediate on all surfaces. Moreover, the ethylene selectivity has a positive correlation with the gallium content by comparing the desorption barrier with the hydrogenation barrier of ethylene, thus the NiGa(111) surface also exhibits the best selectivity. Therefore, the NiGa(111) surface demonstrates to be an excellent reaction facet for the semihydrogenation of acetylene, which agreed with the experimental findings, and would provide helpful instructions for designing and preparing highly-selective and noble-substitute catalysts of alkyne semihydrogenation.
中文翻译:

Ni-Ga金属间化合物催化剂上乙炔加氢的反应机理和选择性:密度泛函理论研究†
金属间化合物(IMCs)对乙炔的选择性加氢显示出优异的催化性能,但是在基于Ni的IMC上对该反应的理论理解相当有限。在这项工作中,通过密度泛函理论(DFT)方法计算了C 2物种的吸附,巴德尔电荷,预计的态密度(PDOS)和反应路径,以研究乙炔上乙炔加氢的机理和选择性。 111)Ni n Ga(n= 1、3)IMC,对原始Ni(111)表面进行了比较研究。结果表明,乙炔的吸附能随着Ni / Ga比的增加而增加,因此,在富Ga的表面上可行的乙炔吸附保证了较低的有效势垒,从而导致三个表面中NiGa(111)表面的最佳活性。不良电荷分析表明,电子从Ga原子转移到Ni原子并进一步传递到C 2物种,与Ni(111)和Ni 3 Ga(111)上的吸附相比,C 2物种在NiGa(111)上的吸附能力降低。。乙炔氢化的反应途径乙烯通过乙烯基或甚至超过氢化为乙烷经由乙基比在所有表面上包含亚乙基中间体的途径都更有利。此外,通过将乙烯的解吸势垒与加氢势垒进行比较,乙烯的选择性与镓含量呈正相关,因此NiGa(111)表面也表现出最佳的选择性。因此,NiGa(111)表面被证明是乙炔半加氢反应的极好反应面,与实验结果相吻合,并将为设计和制备炔烃半加氢反应的高选择性和高取代度催化剂提供有用的指导。
更新日期:2018-02-06
中文翻译:

Ni-Ga金属间化合物催化剂上乙炔加氢的反应机理和选择性:密度泛函理论研究†
金属间化合物(IMCs)对乙炔的选择性加氢显示出优异的催化性能,但是在基于Ni的IMC上对该反应的理论理解相当有限。在这项工作中,通过密度泛函理论(DFT)方法计算了C 2物种的吸附,巴德尔电荷,预计的态密度(PDOS)和反应路径,以研究乙炔上乙炔加氢的机理和选择性。 111)Ni n Ga(n= 1、3)IMC,对原始Ni(111)表面进行了比较研究。结果表明,乙炔的吸附能随着Ni / Ga比的增加而增加,因此,在富Ga的表面上可行的乙炔吸附保证了较低的有效势垒,从而导致三个表面中NiGa(111)表面的最佳活性。不良电荷分析表明,电子从Ga原子转移到Ni原子并进一步传递到C 2物种,与Ni(111)和Ni 3 Ga(111)上的吸附相比,C 2物种在NiGa(111)上的吸附能力降低。。乙炔氢化的反应途径乙烯通过乙烯基或甚至超过氢化为乙烷经由乙基比在所有表面上包含亚乙基中间体的途径都更有利。此外,通过将乙烯的解吸势垒与加氢势垒进行比较,乙烯的选择性与镓含量呈正相关,因此NiGa(111)表面也表现出最佳的选择性。因此,NiGa(111)表面被证明是乙炔半加氢反应的极好反应面,与实验结果相吻合,并将为设计和制备炔烃半加氢反应的高选择性和高取代度催化剂提供有用的指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号