当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Living ring-opening polymerization of ε-caprolactone catalyzed by β-quinolyl-enamino aluminium complexes: ligand electronic effects†
Dalton Transactions ( IF 4 ) Pub Date : 2018-02-06 00:00:00 , DOI: 10.1039/c7dt04522k Peng Wang 1, 2, 3, 4 , Jianbin Chao 2, 4, 5, 6 , Xia Chen 1, 2, 3, 4, 7
Dalton Transactions ( IF 4 ) Pub Date : 2018-02-06 00:00:00 , DOI: 10.1039/c7dt04522k Peng Wang 1, 2, 3, 4 , Jianbin Chao 2, 4, 5, 6 , Xia Chen 1, 2, 3, 4, 7
Affiliation
|
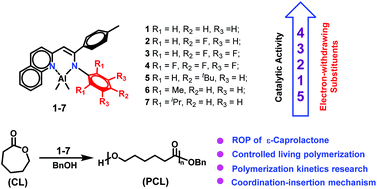
A series of β-quinolyl-enamino aluminium complexes [AlLMe2] 1–7 {L = [(2-C9H6N)-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(4-MeC6H4)-N(Ar)–], Ar = C6H5 (1), 4-FC6H4 (2), 3,4,5-F3C6H2 (3), C6F5 (4), 5-tBuC6H4 (5), 2,6-Me2C6H3 (6), and 2,6-iPr2C6H3 (7)} were synthesized by the reaction of AlMe3 with the corresponding β-quinolyl-enamine ligands. All the complexes were characterized by 1H and 13C NMR spectroscopy and elemental analysis. The molecular structures of complexes 1–3 and 7 were confirmed by single-crystal X-ray diffraction, which revealed that all the Al atoms adopt a distorted tetrahedral geometry. These Al complexes were tested as the initiators for the ring-opening polymerization (ROP) of ε-caprolactone (ε-CL). The polymerization results showed that 1–5 in the presence of BnOH proceeded efficiently and their catalytic behaviors toward the ROP of ε-CL were significantly affected by the substituents of the aryl ring (Ar) on the end of the enamino framework, where the placement of electron-withdrawing substituents caused higher catalytic activity. However 6 and 7 displayed poor activity under the same conditions, suggesting that ortho-substituents on the Ar moieties hamper the coordination–insertion of the ε-CL monomer. The ROP of ε-CL by efficient binary catalytic systems proceeded in a living manner evidenced by the narrow PDIs and the linear nature of Mnversus conversion plots and monomer/catalyst ratios.
C(4-MeC6H4)-N(Ar)–], Ar = C6H5 (1), 4-FC6H4 (2), 3,4,5-F3C6H2 (3), C6F5 (4), 5-tBuC6H4 (5), 2,6-Me2C6H3 (6), and 2,6-iPr2C6H3 (7)} were synthesized by the reaction of AlMe3 with the corresponding β-quinolyl-enamine ligands. All the complexes were characterized by 1H and 13C NMR spectroscopy and elemental analysis. The molecular structures of complexes 1–3 and 7 were confirmed by single-crystal X-ray diffraction, which revealed that all the Al atoms adopt a distorted tetrahedral geometry. These Al complexes were tested as the initiators for the ring-opening polymerization (ROP) of ε-caprolactone (ε-CL). The polymerization results showed that 1–5 in the presence of BnOH proceeded efficiently and their catalytic behaviors toward the ROP of ε-CL were significantly affected by the substituents of the aryl ring (Ar) on the end of the enamino framework, where the placement of electron-withdrawing substituents caused higher catalytic activity. However 6 and 7 displayed poor activity under the same conditions, suggesting that ortho-substituents on the Ar moieties hamper the coordination–insertion of the ε-CL monomer. The ROP of ε-CL by efficient binary catalytic systems proceeded in a living manner evidenced by the narrow PDIs and the linear nature of Mnversus conversion plots and monomer/catalyst ratios.
中文翻译:

β-喹啉基-烯氨基铝配合物催化ε-己内酯的活性开环聚合:配体电子效应†
一系列β-喹啉基-烯氨基铝配合物[AlLMe 2 ] 1–7 {L = [(2-C 9 H 6 N)-CH![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C(4-MeC 6 H 4)-N(Ar)–],Ar = C 6 H 5(1),4-FC 6 H 4(2),3,4,5-F 3 C 6 H 2(3),C 6 F 5(4),5- t BuC 6 H 4(5),2,6-Me 2 C 6 H通过AlMe 3与相应的β-喹啉基-烯胺配体的反应合成了图3( 6)和2,6 - i Pr 2 C 6 H 3( 7)}。所有配合物均通过1 H和13 C NMR光谱学和元素分析表征。配合物1-3和7的分子结构已通过单晶X射线衍射确认,这表明所有Al原子均采用扭曲的四面体几何形状。测试了这些Al络合物作为ε-己内酯(ε-CL)开环聚合(ROP)的引发剂。聚合结果表明1-5在BnOH的存在下有效地进行,并且其朝向ε-CL的ROP催化行为由芳环(Ar)的对烯胺框架的端部,所述取代基分别显著的影响,其中的吸电子取代基的放置引起较高的催化活性。然而,6和7在相同条件下显示出较差的活性,这表明Ar部分上的邻位取代基阻碍了ε-CL单体的配位-插入。高效的二元催化体系对ε-CL的ROP以生动的方式进行,这由窄的PDI和M n与转化率图和单体/催化剂比的线性关系证明。
C(4-MeC 6 H 4)-N(Ar)–],Ar = C 6 H 5(1),4-FC 6 H 4(2),3,4,5-F 3 C 6 H 2(3),C 6 F 5(4),5- t BuC 6 H 4(5),2,6-Me 2 C 6 H通过AlMe 3与相应的β-喹啉基-烯胺配体的反应合成了图3( 6)和2,6 - i Pr 2 C 6 H 3( 7)}。所有配合物均通过1 H和13 C NMR光谱学和元素分析表征。配合物1-3和7的分子结构已通过单晶X射线衍射确认,这表明所有Al原子均采用扭曲的四面体几何形状。测试了这些Al络合物作为ε-己内酯(ε-CL)开环聚合(ROP)的引发剂。聚合结果表明1-5在BnOH的存在下有效地进行,并且其朝向ε-CL的ROP催化行为由芳环(Ar)的对烯胺框架的端部,所述取代基分别显著的影响,其中的吸电子取代基的放置引起较高的催化活性。然而,6和7在相同条件下显示出较差的活性,这表明Ar部分上的邻位取代基阻碍了ε-CL单体的配位-插入。高效的二元催化体系对ε-CL的ROP以生动的方式进行,这由窄的PDI和M n与转化率图和单体/催化剂比的线性关系证明。
更新日期:2018-02-06
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(4-MeC6H4)-N(Ar)–], Ar = C6H5 (1), 4-FC6H4 (2), 3,4,5-F3C6H2 (3), C6F5 (4), 5-tBuC6H4 (5), 2,6-Me2C6H3 (6), and 2,6-iPr2C6H3 (7)} were synthesized by the reaction of AlMe3 with the corresponding β-quinolyl-enamine ligands. All the complexes were characterized by 1H and 13C NMR spectroscopy and elemental analysis. The molecular structures of complexes 1–3 and 7 were confirmed by single-crystal X-ray diffraction, which revealed that all the Al atoms adopt a distorted tetrahedral geometry. These Al complexes were tested as the initiators for the ring-opening polymerization (ROP) of ε-caprolactone (ε-CL). The polymerization results showed that 1–5 in the presence of BnOH proceeded efficiently and their catalytic behaviors toward the ROP of ε-CL were significantly affected by the substituents of the aryl ring (Ar) on the end of the enamino framework, where the placement of electron-withdrawing substituents caused higher catalytic activity. However 6 and 7 displayed poor activity under the same conditions, suggesting that ortho-substituents on the Ar moieties hamper the coordination–insertion of the ε-CL monomer. The ROP of ε-CL by efficient binary catalytic systems proceeded in a living manner evidenced by the narrow PDIs and the linear nature of Mnversus conversion plots and monomer/catalyst ratios.
C(4-MeC6H4)-N(Ar)–], Ar = C6H5 (1), 4-FC6H4 (2), 3,4,5-F3C6H2 (3), C6F5 (4), 5-tBuC6H4 (5), 2,6-Me2C6H3 (6), and 2,6-iPr2C6H3 (7)} were synthesized by the reaction of AlMe3 with the corresponding β-quinolyl-enamine ligands. All the complexes were characterized by 1H and 13C NMR spectroscopy and elemental analysis. The molecular structures of complexes 1–3 and 7 were confirmed by single-crystal X-ray diffraction, which revealed that all the Al atoms adopt a distorted tetrahedral geometry. These Al complexes were tested as the initiators for the ring-opening polymerization (ROP) of ε-caprolactone (ε-CL). The polymerization results showed that 1–5 in the presence of BnOH proceeded efficiently and their catalytic behaviors toward the ROP of ε-CL were significantly affected by the substituents of the aryl ring (Ar) on the end of the enamino framework, where the placement of electron-withdrawing substituents caused higher catalytic activity. However 6 and 7 displayed poor activity under the same conditions, suggesting that ortho-substituents on the Ar moieties hamper the coordination–insertion of the ε-CL monomer. The ROP of ε-CL by efficient binary catalytic systems proceeded in a living manner evidenced by the narrow PDIs and the linear nature of Mnversus conversion plots and monomer/catalyst ratios.
中文翻译:

β-喹啉基-烯氨基铝配合物催化ε-己内酯的活性开环聚合:配体电子效应†
一系列β-喹啉基-烯氨基铝配合物[AlLMe 2 ] 1–7 {L = [(2-C 9 H 6 N)-CH
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C(4-MeC 6 H 4)-N(Ar)–],Ar = C 6 H 5(1),4-FC 6 H 4(2),3,4,5-F 3 C 6 H 2(3),C 6 F 5(4),5- t BuC 6 H 4(5),2,6-Me 2 C 6 H通过AlMe 3与相应的β-喹啉基-烯胺配体的反应合成了图3( 6)和2,6 - i Pr 2 C 6 H 3( 7)}。所有配合物均通过1 H和13 C NMR光谱学和元素分析表征。配合物1-3和7的分子结构已通过单晶X射线衍射确认,这表明所有Al原子均采用扭曲的四面体几何形状。测试了这些Al络合物作为ε-己内酯(ε-CL)开环聚合(ROP)的引发剂。聚合结果表明1-5在BnOH的存在下有效地进行,并且其朝向ε-CL的ROP催化行为由芳环(Ar)的对烯胺框架的端部,所述取代基分别显著的影响,其中的吸电子取代基的放置引起较高的催化活性。然而,6和7在相同条件下显示出较差的活性,这表明Ar部分上的邻位取代基阻碍了ε-CL单体的配位-插入。高效的二元催化体系对ε-CL的ROP以生动的方式进行,这由窄的PDI和M n与转化率图和单体/催化剂比的线性关系证明。
C(4-MeC 6 H 4)-N(Ar)–],Ar = C 6 H 5(1),4-FC 6 H 4(2),3,4,5-F 3 C 6 H 2(3),C 6 F 5(4),5- t BuC 6 H 4(5),2,6-Me 2 C 6 H通过AlMe 3与相应的β-喹啉基-烯胺配体的反应合成了图3( 6)和2,6 - i Pr 2 C 6 H 3( 7)}。所有配合物均通过1 H和13 C NMR光谱学和元素分析表征。配合物1-3和7的分子结构已通过单晶X射线衍射确认,这表明所有Al原子均采用扭曲的四面体几何形状。测试了这些Al络合物作为ε-己内酯(ε-CL)开环聚合(ROP)的引发剂。聚合结果表明1-5在BnOH的存在下有效地进行,并且其朝向ε-CL的ROP催化行为由芳环(Ar)的对烯胺框架的端部,所述取代基分别显著的影响,其中的吸电子取代基的放置引起较高的催化活性。然而,6和7在相同条件下显示出较差的活性,这表明Ar部分上的邻位取代基阻碍了ε-CL单体的配位-插入。高效的二元催化体系对ε-CL的ROP以生动的方式进行,这由窄的PDI和M n与转化率图和单体/催化剂比的线性关系证明。



























 京公网安备 11010802027423号
京公网安备 11010802027423号