当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
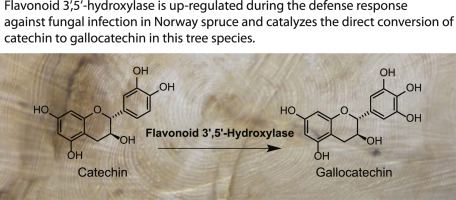
Gallocatechin biosynthesis via a flavonoid 3′,5′-hydroxylase is a defense response in Norway spruce against infection by the bark beetle-associated sap-staining fungus Endoconidiophora polonica
Phytochemistry ( IF 3.8 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.phytochem.2018.01.017 Almuth Hammerbacher , Bettina Raguschke , Louwrance P. Wright , Jonathan Gershenzon
Phytochemistry ( IF 3.8 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.phytochem.2018.01.017 Almuth Hammerbacher , Bettina Raguschke , Louwrance P. Wright , Jonathan Gershenzon
|
One of the best-studied defense responses to fungal infection in Norway spruce (Picea abies) is the biosynthesis of flavan-3-ols, which accumulate as monomers or polymers known as proanthocyanidins. The individual flavan-3-ol units consist of compounds with a 3',4'-dihydroxylated B ring [2,3-(trans)-(+)-catechin or 2,3-(cis)-(-)-epicatechin] and compounds with a 3',4',5'-trihydroxylated B ring [2,3 (trans)-(+)-gallocatechin or 2,3-(cis)-(-)-epigallocatechin]. While much is known about the biosynthesis and biological activity of catechin in Norway spruce, there is little comparable information about gallocatechin or epigallocatechin. We found that there was a significant increase in the gallocatechin content of Norway spruce bark and wood after inoculation with the bark beetle-associated sap-staining fungus Endoconidiophora polonica. Gallocatechins increased proportionally more than catechins as both monomers and units of polymers. A flavonoid 3',5'-hydroxylase gene identified in Norway spruce was shown by heterologous expression in Nicotiana benthamiana to be involved in the conversion of 2,3 (trans)-(+)-catechin to 2,3 (trans)-(+)-gallocatechin. The formation of the trihydroxylated B ring in Norway spruce occurs at the level of flavan-3-ols, rather than at the level of dihydroflavonols as in many angiosperms. The transcript abundance of the flavonoid 3',5'-hydroxylase gene also increased significantly during fungal infection underlining its importance in gallocatechin biosynthesis. Comparisons of the effect of 2,3 (trans)-(+)-catechin and 2,3 (trans)-(+)-gallocatechin on fungal growth revealed that 2,3 (trans)-(+)-catechin is a stronger inhibitor of fungal growth, while 2,3 (trans)-(+)-gallocatechin is a stronger inhibitor of melanin biosynthesis.
中文翻译:

通过类黄酮 3',5'-羟化酶合成没食子茶素是挪威云杉对树皮甲虫相关树液染色真菌 Endoconidiophora polonica 感染的防御反应
对挪威云杉(Picea abies)真菌感染进行的最深入研究的防御反应之一是黄烷-3-醇的生物合成,它以单体或聚合物的形式积累,称为原花青素。单独的 flavan-3-ol 单元由具有 3',4'-二羟基化 B 环 [2,3-(trans)-(+)-catechin 或 2,3-(cis)-(-)-epicatechin 的化合物组成] 和具有 3',4',5'-三羟基化 B 环的化合物 [2,3 (反式)-(+)-没食子儿茶素或 2,3-(顺式)-(-)-表没食子儿茶素]。虽然对挪威云杉中儿茶素的生物合成和生物活性了解很多,但关于没食子儿茶素或表没食子儿茶素的可比信息却很少。我们发现,在接种与树皮甲虫相关的树液染色真菌 Endoconidiophora polonica 后,挪威云杉树皮和木材的没食子儿茶素含量显着增加。作为聚合物的单体和单元,儿茶素的增加比例高于儿茶素。在挪威云杉中鉴定的类黄酮 3',5'-羟化酶基因在本氏烟草中的异源表达表明参与 2,3(反式)-(+)-儿茶素向 2,3(反式)-( +)-没食子儿茶素。挪威云杉中三羟基化 B 环的形成发生在 flavan-3-ols 的水平,而不是像许多被子植物那样在二氢黄酮醇的水平上形成。黄酮类化合物 3',5' 的转录丰度 α-羟化酶基因在真菌感染期间也显着增加,突显了其在没食子儿茶素生物合成中的重要性。2,3 (反式)-(+)-儿茶素和 2,3 (反式)-(+)-没食子儿茶素对真菌生长影响的比较表明,2,3 (反式)-(+)-儿茶素是更强的真菌生长抑制剂,而 2,3 (反式)-(+)-没食子儿茶素是黑色素生物合成的更强抑制剂。
更新日期:2018-04-01
中文翻译:

通过类黄酮 3',5'-羟化酶合成没食子茶素是挪威云杉对树皮甲虫相关树液染色真菌 Endoconidiophora polonica 感染的防御反应
对挪威云杉(Picea abies)真菌感染进行的最深入研究的防御反应之一是黄烷-3-醇的生物合成,它以单体或聚合物的形式积累,称为原花青素。单独的 flavan-3-ol 单元由具有 3',4'-二羟基化 B 环 [2,3-(trans)-(+)-catechin 或 2,3-(cis)-(-)-epicatechin 的化合物组成] 和具有 3',4',5'-三羟基化 B 环的化合物 [2,3 (反式)-(+)-没食子儿茶素或 2,3-(顺式)-(-)-表没食子儿茶素]。虽然对挪威云杉中儿茶素的生物合成和生物活性了解很多,但关于没食子儿茶素或表没食子儿茶素的可比信息却很少。我们发现,在接种与树皮甲虫相关的树液染色真菌 Endoconidiophora polonica 后,挪威云杉树皮和木材的没食子儿茶素含量显着增加。作为聚合物的单体和单元,儿茶素的增加比例高于儿茶素。在挪威云杉中鉴定的类黄酮 3',5'-羟化酶基因在本氏烟草中的异源表达表明参与 2,3(反式)-(+)-儿茶素向 2,3(反式)-( +)-没食子儿茶素。挪威云杉中三羟基化 B 环的形成发生在 flavan-3-ols 的水平,而不是像许多被子植物那样在二氢黄酮醇的水平上形成。黄酮类化合物 3',5' 的转录丰度 α-羟化酶基因在真菌感染期间也显着增加,突显了其在没食子儿茶素生物合成中的重要性。2,3 (反式)-(+)-儿茶素和 2,3 (反式)-(+)-没食子儿茶素对真菌生长影响的比较表明,2,3 (反式)-(+)-儿茶素是更强的真菌生长抑制剂,而 2,3 (反式)-(+)-没食子儿茶素是黑色素生物合成的更强抑制剂。

























 京公网安备 11010802027423号
京公网安备 11010802027423号