当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Trifunctional High-Throughput Screen Identifies Promising Scaffold To Inhibit Grp94 and Treat Myocilin-Associated Glaucoma
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1021/acschembio.7b01083 Dustin J. E. Huard 1 , Vincent M. Crowley 2 , Yuhong Du 3 , Ricardo A. Cordova 4 , Zheying Sun 4 , Moya O. Tomlin 1 , Chad A. Dickey 4 , John Koren 4 , Laura Blair 4 , Haian Fu 3 , Brian S. J. Blagg 5 , Raquel L. Lieberman 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1021/acschembio.7b01083 Dustin J. E. Huard 1 , Vincent M. Crowley 2 , Yuhong Du 3 , Ricardo A. Cordova 4 , Zheying Sun 4 , Moya O. Tomlin 1 , Chad A. Dickey 4 , John Koren 4 , Laura Blair 4 , Haian Fu 3 , Brian S. J. Blagg 5 , Raquel L. Lieberman 1
Affiliation
|
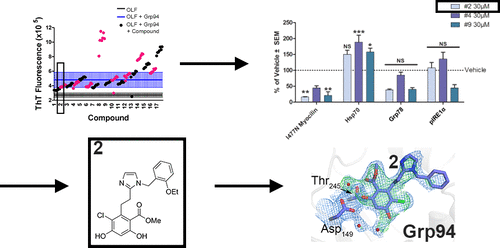
Gain-of-function mutations within the olfactomedin (OLF) domain of myocilin result in its toxic intracellular accumulation and hasten the onset of open-angle glaucoma. The absence of myocilin does not cause disease; therefore, strategies aimed at eliminating myocilin could lead to a successful glaucoma treatment. The endoplasmic reticulum Hsp90 paralog Grp94 accelerates OLF aggregation. Knockdown or pharmacological inhibition of Grp94 in cells facilitates clearance of mutant myocilin via a non-proteasomal pathway. Here, we expanded our support for targeting Grp94 over cytosolic paralogs Hsp90α and Hsp90β. We then developed a high-throughput screening assay to identify new chemical matter capable of disrupting the Grp94/OLF interaction. When applied to a blind, focused library of 17 Hsp90 inhibitors, our miniaturized single-read in vitro thioflavin T -based kinetics aggregation assay exclusively identified compounds that target the chaperone N-terminal nucleotide binding site. In follow up studies, one compound (2) decreased the extent of co-aggregation of Grp94 with OLF in a dose-dependent manner in vitro, and enabled clearance of the aggregation-prone full-length myocilin variant I477N in cells without inducing the heat shock response or causing cytotoxicity. Comparison of the co-crystal structure of compound 2 and another non-selective hit in complex with the N-terminal domain of Grp94 reveals a docking mode tailored to Grp94 and explains its selectivity. A new lead compound has been identified, supporting a targeted chemical biology assay approach to develop a protein degradation-based therapy for myocilin-associated glaucoma by selectively inhibiting Grp94.
中文翻译:

三功能高通量屏幕可识别有望抑制Grp94的支架并治疗与Myocilin相关的青光眼
Myocilin的olfactomedin(OLF)域内的功能获得性突变导致其毒性细胞内积累并加速开角型青光眼的发作。缺乏myocilin不会导致疾病;因此,旨在消除肌球蛋白的策略可能会导致成功的青光眼治疗。内质网Hsp90旁系同源Grp94加速OLF聚集。细胞中Grp94的抑制或药理抑制作用可通过非蛋白酶体途径促进突变型肌球蛋白的清除。在这里,我们扩大了针对Grp94的支持,而不是支持胞质旁系同源物Hsp90α和Hsp90β。然后,我们开发了一种高通量筛选测定法,以鉴定能够破坏Grp94 / OLF相互作用的新化学物质。当应用于17种Hsp90抑制剂的盲目聚焦库时,我们的小型单读体外基于硫黄素T的动力学聚集测定法专门鉴定了靶向伴侣N末端核苷酸结合位点的化合物。在后续研究中,一种化合物(2)在体外以剂量依赖的方式降低了Grp94与OLF的共聚集程度,并在不诱导热量的情况下清除了细胞中易于聚集的全长肌球蛋白变体I477N。休克反应或引起细胞毒性。化合物2的共晶结构比较与Grp94 N末端结构域形成复合体的另一种非选择性命中揭示了针对Grp94的对接模式,并解释了其选择性。已经确定了一种新的先导化合物,该化合物可通过选择性抑制Grp94来支持靶向化学生物学分析方法,以开发一种基于蛋白质降解的治疗肌球蛋白相关性青光眼的疗法。
更新日期:2018-02-05
中文翻译:

三功能高通量屏幕可识别有望抑制Grp94的支架并治疗与Myocilin相关的青光眼
Myocilin的olfactomedin(OLF)域内的功能获得性突变导致其毒性细胞内积累并加速开角型青光眼的发作。缺乏myocilin不会导致疾病;因此,旨在消除肌球蛋白的策略可能会导致成功的青光眼治疗。内质网Hsp90旁系同源Grp94加速OLF聚集。细胞中Grp94的抑制或药理抑制作用可通过非蛋白酶体途径促进突变型肌球蛋白的清除。在这里,我们扩大了针对Grp94的支持,而不是支持胞质旁系同源物Hsp90α和Hsp90β。然后,我们开发了一种高通量筛选测定法,以鉴定能够破坏Grp94 / OLF相互作用的新化学物质。当应用于17种Hsp90抑制剂的盲目聚焦库时,我们的小型单读体外基于硫黄素T的动力学聚集测定法专门鉴定了靶向伴侣N末端核苷酸结合位点的化合物。在后续研究中,一种化合物(2)在体外以剂量依赖的方式降低了Grp94与OLF的共聚集程度,并在不诱导热量的情况下清除了细胞中易于聚集的全长肌球蛋白变体I477N。休克反应或引起细胞毒性。化合物2的共晶结构比较与Grp94 N末端结构域形成复合体的另一种非选择性命中揭示了针对Grp94的对接模式,并解释了其选择性。已经确定了一种新的先导化合物,该化合物可通过选择性抑制Grp94来支持靶向化学生物学分析方法,以开发一种基于蛋白质降解的治疗肌球蛋白相关性青光眼的疗法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号