Journal of Catalysis ( IF 7.3 ) Pub Date : 2018-02-03 , DOI: 10.1016/j.jcat.2017.12.028 Ioannis Katsounaros , Marta C. Figueiredo , Federico Calle-Vallejo , Hongjiao Li , Andrew A. Gewirth , Nenad M. Markovic , Marc T.M. Koper
|
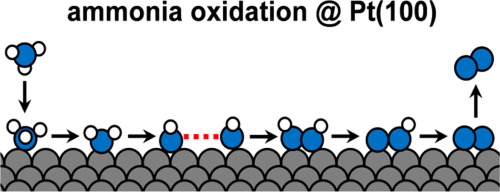
The electrochemical oxidation of ammonia to dinitrogen is a model reaction for the electrocatalysis of the nitrogen cycle, as it can contribute to the understanding of the making/breaking of NN, N
O, or N
H bonds. Moreover, it can be used as the anode reaction in ammonia electrolyzers for H2 production or in ammonia fuel cells. We study here the reaction on the N2-forming Pt(1 0 0) electrode using a combination of electrochemical methods, product characterization and computational methods, and suggest a mechanism that is compatible with the experimental and theoretical findings. We propose that N2 is formed via an ∗NH + ∗NH coupling step, in accordance with the Gerischer-Mauerer mechanism. Other N
N bond-forming steps are considered less likely based on either their unfavourable energetics or the low coverage of the necessary monomers. The N
N coupling is inhibited by strongly adsorbed ∗N and ∗NO species, which are formed by further oxidation of ∗NH.
中文翻译:

碱性环境下氨在Pt(1 0 0)上电化学转化为二氮的机理
氨到二氮的电化学氧化是氮循环电催化的模型反应,因为它有助于理解N N,N
O或N
H键的形成/断裂。此外,它可以在用于生产H 2的氨电解槽中或在氨燃料电池中用作阳极反应。我们在这里使用电化学方法,产物表征和计算方法相结合的方法研究在N 2形成Pt(1 0 0)电极上的反应,并提出与实验和理论发现兼容的机理。我们建议N 2通过∗ NH + ∗形成根据Gerischer-Mauerer机理,进行NH偶联步骤。基于其他N
N键形成步骤,可能是由于它们不利的能量或必需单体的覆盖率较低而被认为不太可能。N
N耦合受到强烈吸附的* N和* NO物种的抑制,这些物质是由* NH的进一步氧化形成的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号