当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aqueous Phase Photo-oxidation of Brown Carbon Nitrophenols: Reaction Kinetics, Mechanism, and Evolution of Light Absorption
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00123 Rachel F. Hems 1 , Jonathan P. D. Abbatt 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00123 Rachel F. Hems 1 , Jonathan P. D. Abbatt 1
Affiliation
|
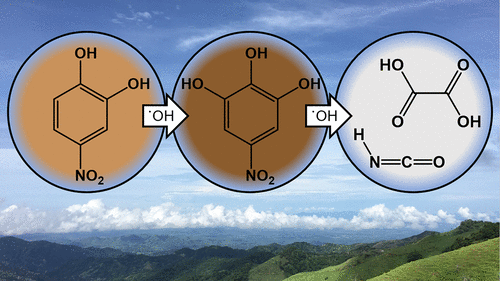
Light absorbing organic aerosol particles, referred to as brown carbon, are geographically widespread and can have an important climate impact through the absorption of solar radiation. Recent studies, both in the laboratory and the field, have shown that brown carbon aerosols can be bleached of their color by direct photolysis and photo-oxidation reactions on the time scale of hours to days. However, the photo-oxidation of nitrophenol molecules, which are colored compounds often associated with biomass burning organic aerosol, show an enhancement in light absorption before the color is lost. This study investigates the mechanism of color enhancement and the fate of three nitrophenol compounds, specifically nitrocatechol, nitroguaiacol, and dinitrophenol, in the aqueous phase using online aerosol chemical ionization mass spectrometry (aerosol-CIMS). The second-order rate constants for the three nitrophenols with OH radicals in the aqueous phase at pH 7 (298 K), were determined to be 5 × 109 M–1 s–1, 5.2 × 109 M–1 s–1, and 3.7 × 109 M–1 s–1 for nitrocatechol, nitroguaiacol, and dinitrophenol, respectively. For a representative aqueous OH concentration, these rate constants correspond to an aqueous lifetime with respect to OH on the order of hours. While the nitrophenol molecules react rapidly with OH, the initial products, which are functionalized by additional electron-donating OH groups, likely lead to the observed absorption increase in the visible range. Further photo-oxidation fragments the aromatic structure to produce smaller, highly oxygenated molecules which no longer absorb strongly at visible wavelengths. These products include furoic acid, glyoxylic acid, malonic acid, oxalic acid, and isocyanic acid. All three nitrophenols investigated formed similar products during photo-oxidation, suggesting that these results could be generalized to this larger class of compounds.
中文翻译:

棕碳硝基酚的水相光氧化:反应动力学,机理和光吸收的演变。
吸收光的有机气溶胶颗粒(称为棕碳)在地理上分布广泛,并且可以通过吸收太阳辐射而对气候产生重要影响。实验室和现场的最新研究表明,在数小时至数天的时间范围内,通过直接的光解和光氧化反应,可以使褐碳气溶胶的颜色漂白。然而,经常与生物质燃烧有机气溶胶有关的有色化合物硝基苯酚分子的光氧化在颜色消失之前显示出光吸收的增强。这项研究探讨了三种硝基苯酚化合物(尤其是硝基邻苯二酚,硝基愈创木酚和二硝基苯酚)的增色机理和命运。在水相中使用在线气溶胶化学电离质谱(aerosol-CIMS)。确定了在pH 7(298 K)下水相中带有OH自由基的三个硝基酚的二阶速率常数为5×109中号-1小号-1,5.2×10 9中号-1小号-1和3.7×10 9中号-1小号-1分别用于硝基儿茶酚,硝基愈创木酚和二硝基苯酚。对于代表性的含水OH浓度,这些速率常数对应于相对于OH的数小时的含水寿命。尽管硝基苯酚分子与OH快速反应,但最初的产物(通过附加的给电子的OH基团官能化)可能导致在可见光范围内观察到的吸收增加。进一步的光氧化使芳族结构断裂,产生较小的,高度氧化的分子,这些分子不再在可见光波长下强烈吸收。这些产品包括糠酸,乙醛酸,丙二酸,草酸和异氰酸。研究的所有三种硝基酚在光氧化过程中形成相似的产物,这表明这些结果可以推广到这类更大的化合物上。
更新日期:2018-01-16
中文翻译:

棕碳硝基酚的水相光氧化:反应动力学,机理和光吸收的演变。
吸收光的有机气溶胶颗粒(称为棕碳)在地理上分布广泛,并且可以通过吸收太阳辐射而对气候产生重要影响。实验室和现场的最新研究表明,在数小时至数天的时间范围内,通过直接的光解和光氧化反应,可以使褐碳气溶胶的颜色漂白。然而,经常与生物质燃烧有机气溶胶有关的有色化合物硝基苯酚分子的光氧化在颜色消失之前显示出光吸收的增强。这项研究探讨了三种硝基苯酚化合物(尤其是硝基邻苯二酚,硝基愈创木酚和二硝基苯酚)的增色机理和命运。在水相中使用在线气溶胶化学电离质谱(aerosol-CIMS)。确定了在pH 7(298 K)下水相中带有OH自由基的三个硝基酚的二阶速率常数为5×109中号-1小号-1,5.2×10 9中号-1小号-1和3.7×10 9中号-1小号-1分别用于硝基儿茶酚,硝基愈创木酚和二硝基苯酚。对于代表性的含水OH浓度,这些速率常数对应于相对于OH的数小时的含水寿命。尽管硝基苯酚分子与OH快速反应,但最初的产物(通过附加的给电子的OH基团官能化)可能导致在可见光范围内观察到的吸收增加。进一步的光氧化使芳族结构断裂,产生较小的,高度氧化的分子,这些分子不再在可见光波长下强烈吸收。这些产品包括糠酸,乙醛酸,丙二酸,草酸和异氰酸。研究的所有三种硝基酚在光氧化过程中形成相似的产物,这表明这些结果可以推广到这类更大的化合物上。



























 京公网安备 11010802027423号
京公网安备 11010802027423号