当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermal Stabilization of Biologics with Photoresponsive Hydrogels
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-02-02 00:00:00 , DOI: 10.1021/acs.biomac.7b01507 Balaji V. Sridhar 1 , John R. Janczy 1 , Øyvind Hatlevik 1 , Gabriel Wolfson 1 , Kristi S. Anseth , Mark W. Tibbitt 2
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-02-02 00:00:00 , DOI: 10.1021/acs.biomac.7b01507 Balaji V. Sridhar 1 , John R. Janczy 1 , Øyvind Hatlevik 1 , Gabriel Wolfson 1 , Kristi S. Anseth , Mark W. Tibbitt 2
Affiliation
|
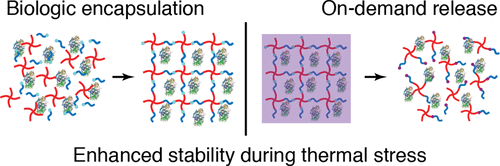
Modern medicine, biological research, and clinical diagnostics depend on the reliable supply and storage of complex biomolecules. However, biomolecules are inherently susceptible to thermal stress and the global distribution of value-added biologics, including vaccines, biotherapeutics, and Research Use Only (RUO) proteins, requires an integrated cold chain from point of manufacture to point of use. To mitigate reliance on the cold chain, formulations have been engineered to protect biologics from thermal stress, including materials-based strategies that impart thermal stability via direct encapsulation of the molecule. While direct encapsulation has demonstrated pronounced stabilization of proteins and complex biological fluids, no solution offers thermal stability while enabling facile and on-demand release from the encapsulating material, a critical feature for broad use. Here we show that direct encapsulation within synthetic, photoresponsive hydrogels protected biologics from thermal stress and afforded user-defined release at the point of use. The poly(ethylene glycol) (PEG)-based hydrogel was formed via a bioorthogonal, click reaction in the presence of biologics without impact on biologic activity. Cleavage of the installed photolabile moiety enabled subsequent dissolution of the network with light and release of the encapsulated biologic. Hydrogel encapsulation improved stability for encapsulated enzymes commonly used in molecular biology (β-galactosidase, alkaline phosphatase, and T4 DNA ligase) following thermal stress. β-galactosidase and alkaline phosphatase were stabilized for 4 weeks at temperatures up to 60 °C, and for 60 min at 85 °C for alkaline phosphatase. T4 DNA ligase, which loses activity rapidly at moderately elevated temperatures, was protected during thermal stress of 40 °C for 24 h and 60 °C for 30 min. These data demonstrate a general method to employ reversible polymer networks as robust excipients for thermal stability of complex biologics during storage and shipment that additionally enable on-demand release of active molecules at the point of use.
中文翻译:

光敏水凝胶对生物制剂的热稳定作用
现代医学,生物学研究和临床诊断取决于复杂生物分子的可靠供应和储存。但是,生物分子固有地容易受到热应力的影响,包括疫苗,生物治疗药物和仅限研究使用(RUO)蛋白质在内的增值生物制剂的全球分布,要求从制造到使用的整个过程都需要一条完整的冷链。为了减轻对冷链的依赖,已对制剂进行了工程设计以保护生物制剂免受热应力的影响,包括通过直接封装分子赋予热稳定性的基于材料的策略。虽然直接封装已证明蛋白质和复杂的生物流体具有明显的稳定性,但没有一种溶液能够提供热稳定性,同时又能从封装材料中轻松按需释放,广泛使用的关键功能。在这里,我们表明在合成的光响应水凝胶中直接包封可保护生物制剂免受热应激和在使用时提供用户定义的发布。基于聚(乙二醇)(PEG)的水凝胶是通过在生物制剂存在下通过生物正交点击反应形成的,而不会影响生物活性。所安装的光不稳定部分的裂解使得网络随后可以用光溶解并释放被包封的生物制剂。水凝胶封装改善了热应激后分子生物学中常用的封装酶(β-半乳糖苷酶,碱性磷酸酶和T4 DNA连接酶)的稳定性。β-半乳糖苷酶和碱性磷酸酶在最高60°C的温度下可稳定4周,而碱性磷酸酶在85°C的温度下可稳定60分钟。T4 DNA连接酶在适度升高的温度下会迅速失去活性,在40°C持续24 h和60°C持续30 min的热应激过程中受到保护。
更新日期:2018-02-02
中文翻译:

光敏水凝胶对生物制剂的热稳定作用
现代医学,生物学研究和临床诊断取决于复杂生物分子的可靠供应和储存。但是,生物分子固有地容易受到热应力的影响,包括疫苗,生物治疗药物和仅限研究使用(RUO)蛋白质在内的增值生物制剂的全球分布,要求从制造到使用的整个过程都需要一条完整的冷链。为了减轻对冷链的依赖,已对制剂进行了工程设计以保护生物制剂免受热应力的影响,包括通过直接封装分子赋予热稳定性的基于材料的策略。虽然直接封装已证明蛋白质和复杂的生物流体具有明显的稳定性,但没有一种溶液能够提供热稳定性,同时又能从封装材料中轻松按需释放,广泛使用的关键功能。在这里,我们表明在合成的光响应水凝胶中直接包封可保护生物制剂免受热应激和在使用时提供用户定义的发布。基于聚(乙二醇)(PEG)的水凝胶是通过在生物制剂存在下通过生物正交点击反应形成的,而不会影响生物活性。所安装的光不稳定部分的裂解使得网络随后可以用光溶解并释放被包封的生物制剂。水凝胶封装改善了热应激后分子生物学中常用的封装酶(β-半乳糖苷酶,碱性磷酸酶和T4 DNA连接酶)的稳定性。β-半乳糖苷酶和碱性磷酸酶在最高60°C的温度下可稳定4周,而碱性磷酸酶在85°C的温度下可稳定60分钟。T4 DNA连接酶在适度升高的温度下会迅速失去活性,在40°C持续24 h和60°C持续30 min的热应激过程中受到保护。



























 京公网安备 11010802027423号
京公网安备 11010802027423号