当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diels–Alder reactions between hexafluoro-2-butyne and bis-furyl dienes: kinetic versus thermodynamic control
Chemical Communications ( IF 4.9 ) Pub Date : 2018-02-02 00:00:00 , DOI: 10.1039/c7cc09466c Kseniya K. Borisova 1, 2, 3, 4, 5 , Eugeniya V. Nikitina 1, 2, 3, 4, 5 , Roman A. Novikov 4, 5, 6, 7 , Victor N. Khrustalev 1, 2, 3, 4, 5 , Pavel V. Dorovatovskii 4, 8, 9 , Yan V. Zubavichus 4, 8, 9 , Maxim L. Kuznetsov 10, 11, 12, 13, 14 , Vladimir P. Zaytsev 1, 2, 3, 4, 5 , Alexey V. Varlamov 1, 2, 3, 4, 5 , Fedor I. Zubkov 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2018-02-02 00:00:00 , DOI: 10.1039/c7cc09466c Kseniya K. Borisova 1, 2, 3, 4, 5 , Eugeniya V. Nikitina 1, 2, 3, 4, 5 , Roman A. Novikov 4, 5, 6, 7 , Victor N. Khrustalev 1, 2, 3, 4, 5 , Pavel V. Dorovatovskii 4, 8, 9 , Yan V. Zubavichus 4, 8, 9 , Maxim L. Kuznetsov 10, 11, 12, 13, 14 , Vladimir P. Zaytsev 1, 2, 3, 4, 5 , Alexey V. Varlamov 1, 2, 3, 4, 5 , Fedor I. Zubkov 1, 2, 3, 4, 5
Affiliation
|
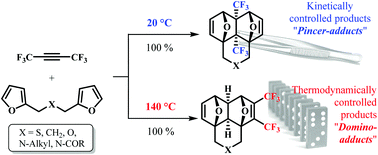
The tandem [4+2] cycloaddition between hexafluoro-2-butyne and bis-furyl dienes, like difurfuryl ester, at room temperature leads to the kinetically controlled “pincer”-adducts – annulated 4a,8a-bis(trifluoromethyl)hexahydro-1,4:5,8-diepoxynaphthalenes. On the other hand, if these reactions proceed at 140 °C, only the thermodynamically controlled “domino”-adducts – annulated 2,3-bis(trifluoromethyl)hexahydro-1,4:5,8-diepoxynaphthalenes – are formed. Therefore, a very rare and unexpected example of full kinetic and thermodynamic control in the Diels–Alder reaction is reported in this paper.
中文翻译:

六氟-2-丁炔与双呋喃二烯之间的狄尔斯-阿尔德反应:动力学与热力学控制
的串联式[4 + 2]六氟-2-丁炔和双-呋喃基二烯烃,如二糠酯,在室温下导致动力学控制“夹钳” -adducts之间环加成-稠4一,8一个双(三氟甲基)六氢-1,4:5,8-双环氧萘。另一方面,如果这些反应在140°C下进行,则仅形成热力学控制的“多米诺”加合物-环化的2,3-双(三氟甲基)六氢-1,4:5,8-双环氧萘。因此,本文报道了狄尔斯-阿尔德反应中完全动力学和热力学控制的非常罕见且出乎意料的例子。
更新日期:2018-03-15
中文翻译:

六氟-2-丁炔与双呋喃二烯之间的狄尔斯-阿尔德反应:动力学与热力学控制
的串联式[4 + 2]六氟-2-丁炔和双-呋喃基二烯烃,如二糠酯,在室温下导致动力学控制“夹钳” -adducts之间环加成-稠4一,8一个双(三氟甲基)六氢-1,4:5,8-双环氧萘。另一方面,如果这些反应在140°C下进行,则仅形成热力学控制的“多米诺”加合物-环化的2,3-双(三氟甲基)六氢-1,4:5,8-双环氧萘。因此,本文报道了狄尔斯-阿尔德反应中完全动力学和热力学控制的非常罕见且出乎意料的例子。


























 京公网安备 11010802027423号
京公网安备 11010802027423号