Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2018-02-02 , DOI: 10.1016/j.bmcl.2018.01.065 Shinya Shiomi , Kohei Wada , Yuhei Umeda , Hikaru Kato , Sachiko Tsukamoto , Hayato Ishikawa
|
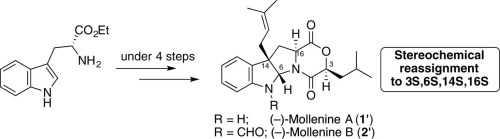
Total syntheses of prenylated pyrrolidinoindoline alkaloids, (−)-mollenines A [(−)-1′] and B (2′), were accomplished via three- and four-step sequences including a bioinspired indole prenylation reaction followed by dioxomorpholine ring formation. Then, the stereochemistry of mollenines A and B was reassigned to 3S,6S,14S,16S by analysis of spectroscopic data and chemical syntheses with different approaches along with the comparison of calculated and experimental ECD spectra. In addition, a thermodynamically controlled epimerization reaction on the dioxomorpholine ring was observed in our synthesis.
中文翻译:

莫来宁A和B的总合成和立体化学重新分配
通过三步和四步序列,包括生物启发的吲哚烯丙基异戊二烯化反应,然后形成二氧代吗啉环,完成了烯丙基化吡咯烷二氢吲哚啉生物碱(-)-mollenines A [(-)- 1 ']和B(2 ')的总合成。然后,通过光谱数据分析和化学合成,采用不同的方法,并比较计算出的和实验的ECD光谱,将亚甲基苯甲酸酯A和B的立体化学重新分配为3 S,6 S,14 S,16 S。另外,在我们的合成中观察到了对二氧代吗啉环的热力学控制的差向异构反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号