当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of α‐(Trifluoromethyl)pyridazine Derivatives
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-02-02 , DOI: 10.1002/ejoc.201701412 Alexandra Feraldi-Xypolia 1 , Domingo Gomez Pardo 1 , Janine Cossy 1
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-02-02 , DOI: 10.1002/ejoc.201701412 Alexandra Feraldi-Xypolia 1 , Domingo Gomez Pardo 1 , Janine Cossy 1
Affiliation
|
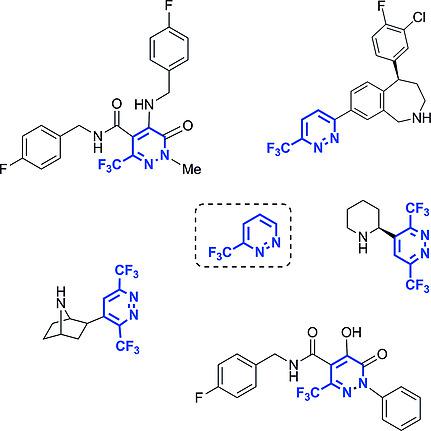
Over the last decades, an increasing interest in α‐(trifluoromethyl)pyridazines in medicinal chemistry has been noticed. This interest stems firstly from the recent development of biologically active pyridazines as well as from the capacity of the pyridazine ring to be a bioisoster of other heterocycles. Secondly, due to the influence of a fluorine atom on the chemical and physico‐chemical properties of organic compounds, fluorinated pyridazines have been an attractive scaffold for medicinal chemists. Consequently, the methods that allow the synthesis of fluorinated pyridazines, and more specifically pyridazines possessing either one or two CF3 groups α to the nitrogen atoms, are of great interest and are reported in this review.
中文翻译:

α-(三氟甲基)哒嗪衍生物的合成
在过去的几十年中,人们已经注意到人们对药用化学中的α-(三氟甲基)哒嗪的兴趣与日俱增。这种兴趣首先源于生物活性哒嗪的最新发展以及哒嗪环作为其他杂环的生物等排体的能力。其次,由于氟原子对有机化合物的化学和物理化学性质的影响,氟化哒嗪已成为药物化学家的一个有吸引力的支架。因此,允许合成氟化哒嗪,尤其是具有一个或两个与氮原子相连的CF 3基团α的哒嗪的方法引起了人们的广泛兴趣,并在本综述中进行了报道。
更新日期:2018-06-03
中文翻译:

α-(三氟甲基)哒嗪衍生物的合成
在过去的几十年中,人们已经注意到人们对药用化学中的α-(三氟甲基)哒嗪的兴趣与日俱增。这种兴趣首先源于生物活性哒嗪的最新发展以及哒嗪环作为其他杂环的生物等排体的能力。其次,由于氟原子对有机化合物的化学和物理化学性质的影响,氟化哒嗪已成为药物化学家的一个有吸引力的支架。因此,允许合成氟化哒嗪,尤其是具有一个或两个与氮原子相连的CF 3基团α的哒嗪的方法引起了人们的广泛兴趣,并在本综述中进行了报道。


























 京公网安备 11010802027423号
京公网安备 11010802027423号