Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Situ Structure of Neuronal C9orf72 Poly-GA Aggregates Reveals Proteasome Recruitment.
Cell ( IF 64.5 ) Pub Date : 2018-Feb-08 , DOI: 10.1016/j.cell.2017.12.030 Qiang Guo 1 , Carina Lehmer 2 , Antonio Martínez-Sánchez 1 , Till Rudack 3 , Florian Beck 1 , Hannelore Hartmann 2 , Manuela Pérez-Berlanga 4 , Frédéric Frottin 4 , Mark S Hipp 5 , F Ulrich Hartl 5 , Dieter Edbauer 6 , Wolfgang Baumeister 1 , Rubén Fernández-Busnadiego 1
Cell ( IF 64.5 ) Pub Date : 2018-Feb-08 , DOI: 10.1016/j.cell.2017.12.030 Qiang Guo 1 , Carina Lehmer 2 , Antonio Martínez-Sánchez 1 , Till Rudack 3 , Florian Beck 1 , Hannelore Hartmann 2 , Manuela Pérez-Berlanga 4 , Frédéric Frottin 4 , Mark S Hipp 5 , F Ulrich Hartl 5 , Dieter Edbauer 6 , Wolfgang Baumeister 1 , Rubén Fernández-Busnadiego 1
Affiliation
|
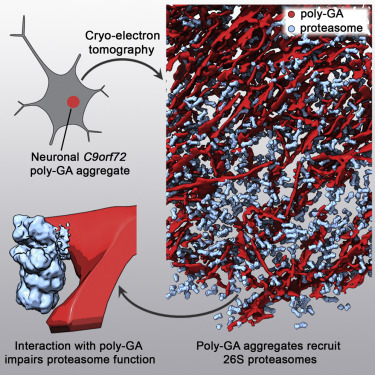
Protein aggregation and dysfunction of the ubiquitin-proteasome system are hallmarks of many neurodegenerative diseases. Here, we address the elusive link between these phenomena by employing cryo-electron tomography to dissect the molecular architecture of protein aggregates within intact neurons at high resolution. We focus on the poly-Gly-Ala (poly-GA) aggregates resulting from aberrant translation of an expanded GGGGCC repeat in C9orf72, the most common genetic cause of amyotrophic lateral sclerosis and frontotemporal dementia. We find that poly-GA aggregates consist of densely packed twisted ribbons that recruit numerous 26S proteasome complexes, while other macromolecules are largely excluded. Proximity to poly-GA ribbons stabilizes a transient substrate-processing conformation of the 26S proteasome, suggesting stalled degradation. Thus, poly-GA aggregates may compromise neuronal proteostasis by driving the accumulation and functional impairment of a large fraction of cellular proteasomes.
中文翻译:

神经元 C9orf72 Poly-GA 聚集体的原位结构揭示了蛋白酶体的募集。
泛素-蛋白酶体系统的蛋白质聚集和功能障碍是许多神经退行性疾病的标志。在这里,我们通过采用低温电子断层扫描技术以高分辨率剖析完整神经元内蛋白质聚集体的分子结构,从而解决这些现象之间难以捉摸的联系。我们专注于由 C9orf72 中扩展的 GGGGCC 重复序列的异常翻译产生的 poly-Gly-Ala (poly-GA) 聚集体,这是肌萎缩侧索硬化和额颞叶痴呆的最常见遗传原因。我们发现 poly-GA 聚集体由密集的扭曲带组成,这些带状结构吸收了许多 26S 蛋白酶体复合物,而其他大分子在很大程度上被排除在外。靠近 poly-GA 带可以稳定 26S 蛋白酶体的瞬时底物加工构象,表明降解停滞。因此,
更新日期:2018-02-01
中文翻译:

神经元 C9orf72 Poly-GA 聚集体的原位结构揭示了蛋白酶体的募集。
泛素-蛋白酶体系统的蛋白质聚集和功能障碍是许多神经退行性疾病的标志。在这里,我们通过采用低温电子断层扫描技术以高分辨率剖析完整神经元内蛋白质聚集体的分子结构,从而解决这些现象之间难以捉摸的联系。我们专注于由 C9orf72 中扩展的 GGGGCC 重复序列的异常翻译产生的 poly-Gly-Ala (poly-GA) 聚集体,这是肌萎缩侧索硬化和额颞叶痴呆的最常见遗传原因。我们发现 poly-GA 聚集体由密集的扭曲带组成,这些带状结构吸收了许多 26S 蛋白酶体复合物,而其他大分子在很大程度上被排除在外。靠近 poly-GA 带可以稳定 26S 蛋白酶体的瞬时底物加工构象,表明降解停滞。因此,



























 京公网安备 11010802027423号
京公网安备 11010802027423号