当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reversible anionic redox activity in Na3RuO4 cathodes: a prototype Na-rich layered oxide†
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2018-01-26 00:00:00 , DOI: 10.1039/c7ee03554c Yu Qiao 1, 2, 3, 4, 5 , Shaohua Guo 6, 7, 8, 9, 10 , Kai Zhu 1, 2, 3, 4 , Pan Liu 11, 12, 13, 14, 15 , Xiang Li 1, 2, 3, 4, 5 , Kezhu Jiang 6, 7, 8, 9, 10 , Cheng-Jun Sun 16, 17, 18, 19 , Mingwei Chen 11, 12, 13, 14, 15 , Haoshen Zhou 6, 7, 8, 9, 10
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2018-01-26 00:00:00 , DOI: 10.1039/c7ee03554c Yu Qiao 1, 2, 3, 4, 5 , Shaohua Guo 6, 7, 8, 9, 10 , Kai Zhu 1, 2, 3, 4 , Pan Liu 11, 12, 13, 14, 15 , Xiang Li 1, 2, 3, 4, 5 , Kezhu Jiang 6, 7, 8, 9, 10 , Cheng-Jun Sun 16, 17, 18, 19 , Mingwei Chen 11, 12, 13, 14, 15 , Haoshen Zhou 6, 7, 8, 9, 10
Affiliation
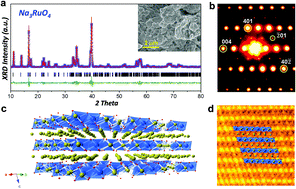
|
Sodium-ion batteries are attractive for large-scale energy storage due to the abundance of sodium, but the deficient capacity achieved by cathode materials prevents their further applications. Chemical substitution of Na in transition metal layers is a promising solution to utilize both the cationic and anionic redox activities for boosting energy storage. Unfortunately, different from the classic Li-rich Li2MnO3, a pure prototype with anionic redox activity has not been found among the typical Na-rich cathodes. Herein, we originally design a Na-rich layered oxide prototype, namely Na3RuO4 (Ru5+), which delivers a partial reversible capacity solely via the participation of oxygen anions. More importantly, the anionic redox activity is validated by the in situ Raman observation of reversible peroxo-based O–O (de)bonding upon cycling. Our findings not only highlight the multiple electron-transfer strategy for capacity extension, but also broaden the horizon in designing Na-rich electrode materials for high-energy sodium-ion batteries.
中文翻译:

Na 3 RuO 4阴极中可逆的阴离子氧化还原活性:富钠原型层状氧化物†
钠离子丰富,因此钠离子电池对于大规模储能具有吸引力,但是阴极材料的容量不足会阻止其进一步应用。在过渡金属层中化学取代Na是一种有前途的解决方案,可以利用阳离子和阴离子氧化还原活性来增强能量存储。不幸的是,不同于经典的富含Li的Li 2 MnO 3,在典型的富含Na的阴极中尚未发现具有阴离子氧化还原活性的纯原型。在此,我们最初设计了一种富含Na的分层氧化物原型,即Na 3 RuO 4(Ru 5+),该原型仅通过以下方式即可提供部分可逆容量:氧阴离子的参与。更重要的是,阴离子的氧化还原活性通过原位拉曼观察到的可循环的过氧基O-O(脱键)结合得到了验证。我们的发现不仅突显了用于扩展容量的多种电子转移策略,而且为设计用于高能钠离子电池的富钠电极材料开阔了眼界。
更新日期:2018-01-26
中文翻译:

Na 3 RuO 4阴极中可逆的阴离子氧化还原活性:富钠原型层状氧化物†
钠离子丰富,因此钠离子电池对于大规模储能具有吸引力,但是阴极材料的容量不足会阻止其进一步应用。在过渡金属层中化学取代Na是一种有前途的解决方案,可以利用阳离子和阴离子氧化还原活性来增强能量存储。不幸的是,不同于经典的富含Li的Li 2 MnO 3,在典型的富含Na的阴极中尚未发现具有阴离子氧化还原活性的纯原型。在此,我们最初设计了一种富含Na的分层氧化物原型,即Na 3 RuO 4(Ru 5+),该原型仅通过以下方式即可提供部分可逆容量:氧阴离子的参与。更重要的是,阴离子的氧化还原活性通过原位拉曼观察到的可循环的过氧基O-O(脱键)结合得到了验证。我们的发现不仅突显了用于扩展容量的多种电子转移策略,而且为设计用于高能钠离子电池的富钠电极材料开阔了眼界。


























 京公网安备 11010802027423号
京公网安备 11010802027423号