当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facilitated Unbinding via Multivalency-Enabled Ternary Complexes: New Paradigm for Protein–DNA Interactions
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1021/acs.accounts.7b00541 Tai-Yen Chen 1 , Yu-Shan Cheng 1 , Pei-San Huang 1 , Peng Chen 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1021/acs.accounts.7b00541 Tai-Yen Chen 1 , Yu-Shan Cheng 1 , Pei-San Huang 1 , Peng Chen 2
Affiliation
|
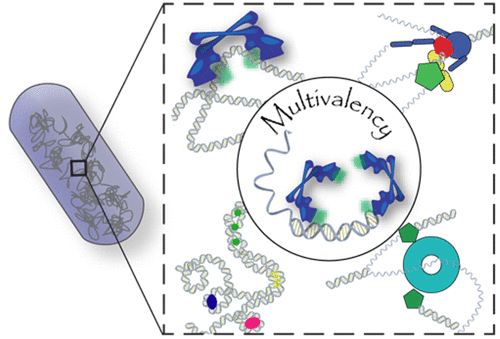
Dynamic protein–DNA interactions constitute highly robust cellular machineries to fulfill cellular functions. A vast number of studies have focused on how DNA-binding proteins search for and interact with their target DNA segments and on what cellular cues can regulate protein binding, for which protein concentration is a most obvious one. In contrast, how protein unbinding could be regulated by protein concentration has evaded attention because protein unbinding from DNA is typically a unimolecular reaction and thus concentration independent. Recent single-molecule studies from multiple research groups have uncovered that protein concentration can facilitate the unbinding of DNA-bound proteins, revealing regulation of protein unbinding as another mechanistic paradigm for gene regulation.
中文翻译:

通过启用多价态的三元复合物促进脱结合:蛋白质-DNA相互作用的新范式
动态的蛋白质与DNA相互作用构成了高度健壮的细胞机制,可以履行细胞功能。大量研究集中在DNA结合蛋白如何搜索其靶DNA片段并与之相互作用,以及哪些细胞信号可以调节蛋白结合,其中最明显的是蛋白浓度。相反,由于蛋白质与DNA的结合通常是单分子反应,因此与浓度无关,因此如何通过蛋白质浓度调节蛋白质的结合便引起人们的关注。来自多个研究小组的最新单分子研究发现,蛋白质浓度可以促进与DNA结合的蛋白质的解除结合,从而揭示蛋白质解除结合的调控是基因调控的另一种机制范式。
更新日期:2018-01-25
中文翻译:

通过启用多价态的三元复合物促进脱结合:蛋白质-DNA相互作用的新范式
动态的蛋白质与DNA相互作用构成了高度健壮的细胞机制,可以履行细胞功能。大量研究集中在DNA结合蛋白如何搜索其靶DNA片段并与之相互作用,以及哪些细胞信号可以调节蛋白结合,其中最明显的是蛋白浓度。相反,由于蛋白质与DNA的结合通常是单分子反应,因此与浓度无关,因此如何通过蛋白质浓度调节蛋白质的结合便引起人们的关注。来自多个研究小组的最新单分子研究发现,蛋白质浓度可以促进与DNA结合的蛋白质的解除结合,从而揭示蛋白质解除结合的调控是基因调控的另一种机制范式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号