当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Renaissance of Sandmeyer-Type Reactions: Conversion of Aromatic C–N Bonds into C–X Bonds (X = B, Sn, P, or CF3)
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1021/acs.accounts.7b00566 Fanyang Mo 1, 2 , Di Qiu 2, 3 , Yan Zhang 2 , Jianbo Wang 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1021/acs.accounts.7b00566 Fanyang Mo 1, 2 , Di Qiu 2, 3 , Yan Zhang 2 , Jianbo Wang 2
Affiliation
|
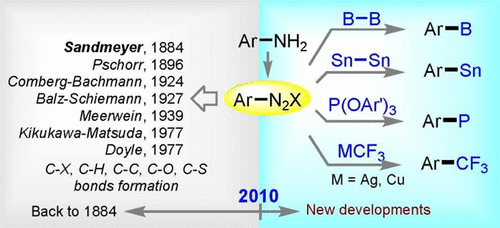
The Sandmeyer reaction represents an important organic transformation that converts an arylamine to an aryl halide using Cu(I) halide via a diazonium salt intermediate. The reaction was first reported by Sandmeyer in 1884, and a number of named reactions closely related to it have been developed and widely applied in organic synthesis throughout the 20th century. These include the Pschorr reaction for the synthesis of biaryl tricycles, the Gomberg–Bachmann reaction for biaryl formations, the Balz–Schiemann reaction for C–F bond formations, and the Meerwein reaction for arylation of α,β-unsaturated carbonyl compounds. However, all these reactions were discovered before 1940. In 1977, Doyle and co-workers reported an organic phase diazotization process, and Kikukawa and Matsuda used aryldiazonium salts in transition metal-catalyzed cross-coupling reactions. However, completely new processes involving diazonium salts have been seldom reported since then, although aryldiazonium salts are widely utilized in modern organic synthesis.
中文翻译:

桑德迈型反应的复兴:芳香C–N键转换为C–X键(X = B,Sn,P或CF 3)
桑德迈尔反应代表了重要的有机转化过程,该过程通过重氮盐中间体使用卤化亚铜(I)将芳基胺转化为芳基卤化物。桑德迈尔(Sandmeyer)于1884年首次报道了该反应,并在20世纪开发了许多与其密切相关的命名反应,并将其广泛应用于有机合成中。其中包括用于合成联芳基三环的Pschorr反应,用于联芳基形成的Gomberg-Bachmann反应,用于C-F键形成的Balz-Schiemann反应以及用于α,β-不饱和羰基化合物芳基化的Meerwein反应。但是,所有这些反应都是在1940年之前发现的。1977年,Doyle及其同事报道了有机相重氮化过程,Kikukawa和Matsuda在过渡金属催化的交叉偶联反应中使用了芳基重氮盐。然而,自那时以来很少报道涉及重氮盐的全新方法,尽管芳基重氮盐被广泛用于现代有机合成中。
更新日期:2018-01-25
中文翻译:

桑德迈型反应的复兴:芳香C–N键转换为C–X键(X = B,Sn,P或CF 3)
桑德迈尔反应代表了重要的有机转化过程,该过程通过重氮盐中间体使用卤化亚铜(I)将芳基胺转化为芳基卤化物。桑德迈尔(Sandmeyer)于1884年首次报道了该反应,并在20世纪开发了许多与其密切相关的命名反应,并将其广泛应用于有机合成中。其中包括用于合成联芳基三环的Pschorr反应,用于联芳基形成的Gomberg-Bachmann反应,用于C-F键形成的Balz-Schiemann反应以及用于α,β-不饱和羰基化合物芳基化的Meerwein反应。但是,所有这些反应都是在1940年之前发现的。1977年,Doyle及其同事报道了有机相重氮化过程,Kikukawa和Matsuda在过渡金属催化的交叉偶联反应中使用了芳基重氮盐。然而,自那时以来很少报道涉及重氮盐的全新方法,尽管芳基重氮盐被广泛用于现代有机合成中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号