当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development and Scale-Up of a Manufacturing Route for the Non-nucleoside Reverse Transcriptase Inhibitor GSK2248761A (IDX-899): Synthesis of an Advanced Key Chiral Intermediate
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1021/acs.oprd.7b00356 Richard Bellingham 1 , Gary Borrett 1 , Guillaume Bret 1 , Bernadette Choudary 1 , David Colclough 1 , Jerome Hayes 1 , John Hayler 1 , Neil Hodnett 1 , Alan Ironmonger 1 , Augustine Ochen 1 , David Pascoe 1 , John Richardson 1 , Erica Vit 1 , François-René Alexandre 2 , Catherine Caillet 2 , Agnès Amador 2 , Stéphanie Bot 2 , Séverine Bonaric 2 , Daniel da Costa 2 , Marie-Pierre Lioure 2 , Arlène Roland 2 , Elodie Rosinovsky 2 , Christophe Parsy 2 , Cyril B. Dousson 2
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1021/acs.oprd.7b00356 Richard Bellingham 1 , Gary Borrett 1 , Guillaume Bret 1 , Bernadette Choudary 1 , David Colclough 1 , Jerome Hayes 1 , John Hayler 1 , Neil Hodnett 1 , Alan Ironmonger 1 , Augustine Ochen 1 , David Pascoe 1 , John Richardson 1 , Erica Vit 1 , François-René Alexandre 2 , Catherine Caillet 2 , Agnès Amador 2 , Stéphanie Bot 2 , Séverine Bonaric 2 , Daniel da Costa 2 , Marie-Pierre Lioure 2 , Arlène Roland 2 , Elodie Rosinovsky 2 , Christophe Parsy 2 , Cyril B. Dousson 2
Affiliation
|
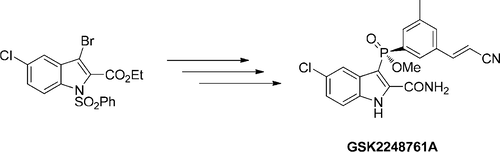
A new and improved synthetic route to an intermediate in the synthesis of the phosphinate ester GSK2248761A is described. In the key step, we describe the first process-scale example of a palladium-catalyzed phosphorus–carbon coupling to give the entire backbone of GSK2248761A in one telescoped stage in 65% average yield on a 68 kg scale. This unusual chemistry enabled the route to be reduced from six chemistry stages to four and eliminated a number of environmentally unfriendly reagents and solvents.
中文翻译:

非核苷逆转录酶抑制剂GSK2248761A(IDX-899)的生产路线的开发和规模扩大:先进的关键手性中间体的合成
描述了合成次膦酸酯GSK2248761A的中间体的新的和改进的合成途径。在关键步骤中,我们描述了钯催化的磷-碳偶联的第一个工艺规模示例,该阶段可在一个伸缩阶段将GSK2248761A的整个主链以68 kg的平均产率达到65%。这种非同寻常的化学反应使路线从六个化学阶段减少到四个,并消除了许多对环境不利的试剂和溶剂。
更新日期:2018-01-25
中文翻译:

非核苷逆转录酶抑制剂GSK2248761A(IDX-899)的生产路线的开发和规模扩大:先进的关键手性中间体的合成
描述了合成次膦酸酯GSK2248761A的中间体的新的和改进的合成途径。在关键步骤中,我们描述了钯催化的磷-碳偶联的第一个工艺规模示例,该阶段可在一个伸缩阶段将GSK2248761A的整个主链以68 kg的平均产率达到65%。这种非同寻常的化学反应使路线从六个化学阶段减少到四个,并消除了许多对环境不利的试剂和溶剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号