当前位置:
X-MOL 学术
›
Toxicol. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Screening for human urinary bladder carcinogens: two-year bioassay is unnecessary†
Toxicology Research ( IF 2.1 ) Pub Date : 2018-01-23 00:00:00 , DOI: 10.1039/c7tx00294g Samuel M. Cohen 1, 2, 3, 4, 5
Toxicology Research ( IF 2.1 ) Pub Date : 2018-01-23 00:00:00 , DOI: 10.1039/c7tx00294g Samuel M. Cohen 1, 2, 3, 4, 5
Affiliation
|
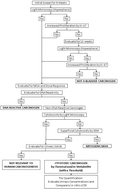
Screening for carcinogens in general, and for the urinary bladder specifically, traditionally involves a two-year bioassay in rodents, the results of which often do not have direct relevance to humans with respect to mode of action (MOA) and/or dose response. My proposal describes a multi-step short-term (90 day) screening process that characterizes known human urinary bladder carcinogens, and identifies those reported in rodent two-year bioassays. The initial step is screening for urothelial proliferation, by microscopy or by increased Ki-67 labeling index. If these are negative, the agent is not a urinary bladder carcinogen. If either of these is positive, an MOA and dose response analysis are performed. DNA reactivity is evaluated. If the chemical is non-DNA reactive, evaluation for cytotoxicity is performed. This involves examination of the urothelium and urine, the latter to identify the generation of urinary solids (e.g. calculi). If urinary solids are the cause of cytotoxicity, the MOA is not relevant to human cancer, but dose response becomes essential for evaluating potential toxicity to humans. If cytotoxicity occurs but no urinary solids are detected, urinary concentrations of the chemical and its metabolites are evaluated, and compared to in vitro cytotoxicity against rodent and human immortalized urothelial cell lines. Based on this process, a screen for urinary bladder carcinogenicity is reliable, and more importantly, can be based on MOA and dose response analyses useful in the overall risk assessment for possible human bladder cancer. The proposed procedure is shorter, less expensive and more relevant than the two-year bioassay.
中文翻译:

筛查人类膀胱致癌物:两年的生物测定是不必要的†
通常,对致癌物进行筛查,特别是对膀胱进行筛查,传统上涉及在啮齿动物中进行为期两年的生物测定,其结果在作用方式(MOA)和/或剂量反应方面通常与人类没有直接关系。我的建议描述了一个多步骤的短期(90天)筛选过程,该过程可表征已知的人类膀胱致癌物,并鉴定出在啮齿动物两年生物测定中报告的那些。第一步是通过显微镜检查或通过增加的Ki-67标记指数筛选尿路上皮的增殖。如果这些结果均为阴性,则说明该药物不是膀胱癌致癌物。如果其中任何一个为阳性,则执行MOA和剂量响应分析。评估DNA反应性。如果该化学品对DNA无反应性,则进行细胞毒性评估。例如结石)。如果尿固形物是细胞毒性的原因,则MOA与人类癌症无关,但是剂量反应对于评估对人类的潜在毒性至关重要。如果发生细胞毒性但未检测到尿液固体,则评估尿液中的化学物质及其代谢产物的浓度,并将其与对啮齿动物和人类永生化尿路上皮细胞系的体外细胞毒性进行比较。基于此过程,对膀胱致癌性的筛查是可靠的,而且更重要的是,可以基于MOA和剂量响应分析,这些分析可用于可能的人类膀胱癌的总体风险评估。所提出的程序比两年的生物测定法更短,更便宜并且更相关。
更新日期:2018-01-23
中文翻译:

筛查人类膀胱致癌物:两年的生物测定是不必要的†
通常,对致癌物进行筛查,特别是对膀胱进行筛查,传统上涉及在啮齿动物中进行为期两年的生物测定,其结果在作用方式(MOA)和/或剂量反应方面通常与人类没有直接关系。我的建议描述了一个多步骤的短期(90天)筛选过程,该过程可表征已知的人类膀胱致癌物,并鉴定出在啮齿动物两年生物测定中报告的那些。第一步是通过显微镜检查或通过增加的Ki-67标记指数筛选尿路上皮的增殖。如果这些结果均为阴性,则说明该药物不是膀胱癌致癌物。如果其中任何一个为阳性,则执行MOA和剂量响应分析。评估DNA反应性。如果该化学品对DNA无反应性,则进行细胞毒性评估。例如结石)。如果尿固形物是细胞毒性的原因,则MOA与人类癌症无关,但是剂量反应对于评估对人类的潜在毒性至关重要。如果发生细胞毒性但未检测到尿液固体,则评估尿液中的化学物质及其代谢产物的浓度,并将其与对啮齿动物和人类永生化尿路上皮细胞系的体外细胞毒性进行比较。基于此过程,对膀胱致癌性的筛查是可靠的,而且更重要的是,可以基于MOA和剂量响应分析,这些分析可用于可能的人类膀胱癌的总体风险评估。所提出的程序比两年的生物测定法更短,更便宜并且更相关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号