当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploration of C–H Transformations of Aldehyde Hydrazones: Radical Strategies and Beyond
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-01-23 00:00:00 , DOI: 10.1021/acs.accounts.7b00565 Pan Xu 1 , Weipeng Li 1 , Jin Xie 1 , Chengjian Zhu 1, 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-01-23 00:00:00 , DOI: 10.1021/acs.accounts.7b00565 Pan Xu 1 , Weipeng Li 1 , Jin Xie 1 , Chengjian Zhu 1, 2
Affiliation
|
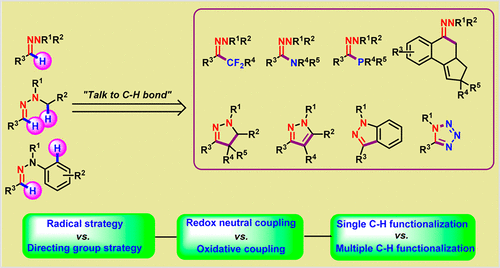
The chemistry of hydrazones has gained great momentum due to their involvement throughout the evolution of organic synthesis. Herein, we discuss the tremendous developments in both the methodology and application of hydrazones. Hydrazones can be recognized not only as synthetic equivalents to aldehydes and ketones but also as versatile synthetic building blocks. Consequently, they can participate in a range of practical synthetic transformations. Furthermore, hydrazone derivatives display a broad array of biological activities and have been widely applied as pharmaceuticals. Owing to the weak directing group effect of simple aldehydes and ketones in C–H bond functionalizations, the C–H bond functionalizations of hydrazones that have been developed in the past five years represent a significant step forward. These novel transformations open a new door to a broader library of functionalized and complex small molecules. Moreover, a wide range of biologically important N-heterocycles (dihydropyrazoles, pyrazoles, indazoles, cinnolines, etc.) can be efficiently synthesized in an atom- and step-economical manner through single, double, or triple C–H bond functionalizations of hydrazones. Both radical C–H functionalizations and transition-metal-catalyzed directing-group strategies have enhanced the synthetic utility of hydrazones in the chemical community because these strategies solve the long-standing challenge of C–H functionalizations adjacent to aldehydes and ketones.
中文翻译:

醛Hy的C–H转换探索:自由基策略及其他
由于hydr在整个有机合成过程中的参与,of的化学获得了巨大的发展势头。在这里,我们讨论的方法学和应用方面的巨大发展。dra不仅可以被认为是醛和酮的合成等同物,而且可以被认为是通用的合成构件。因此,他们可以参与一系列实际的综合转化。此外,衍生物表现出广泛的生物学活性,并已被广泛地用作药物。由于简单醛和酮在C–H键官能化中的弱导向基团作用,近五年来开发的的C–H键官能化代表了向前迈出的重要一步。这些新颖的转变为功能化和复杂的小分子的更广泛的资料库打开了一扇新的大门。而且,广泛的生物学意义N-杂环(二氢吡唑,吡唑,吲唑,cinnolines等)可以通过,的单,双或三键结合以原子经济和分步经济的方式高效合成。自由基C–H官能化和过渡金属催化的导向基团策略都增强了在化学界的合成效用,因为这些策略解决了与醛和酮相邻的C–H官能化的长期挑战。
更新日期:2018-01-23
中文翻译:

醛Hy的C–H转换探索:自由基策略及其他
由于hydr在整个有机合成过程中的参与,of的化学获得了巨大的发展势头。在这里,我们讨论的方法学和应用方面的巨大发展。dra不仅可以被认为是醛和酮的合成等同物,而且可以被认为是通用的合成构件。因此,他们可以参与一系列实际的综合转化。此外,衍生物表现出广泛的生物学活性,并已被广泛地用作药物。由于简单醛和酮在C–H键官能化中的弱导向基团作用,近五年来开发的的C–H键官能化代表了向前迈出的重要一步。这些新颖的转变为功能化和复杂的小分子的更广泛的资料库打开了一扇新的大门。而且,广泛的生物学意义N-杂环(二氢吡唑,吡唑,吲唑,cinnolines等)可以通过,的单,双或三键结合以原子经济和分步经济的方式高效合成。自由基C–H官能化和过渡金属催化的导向基团策略都增强了在化学界的合成效用,因为这些策略解决了与醛和酮相邻的C–H官能化的长期挑战。



























 京公网安备 11010802027423号
京公网安备 11010802027423号