当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Steric and Acidity Control in Hydrogen Bonding and Proton Transfer to trans-W(N2)2(dppe)2
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.inorgchem.7b03027 Nikolay V. Kireev 1, 2 , Oleg A. Filippov 1 , Alexander A. Pavlov 1 , Lina M. Epstein 1 , Victor D. Makhaev 3 , Victor P. Dyadchenko 2 , Elena S. Shubina 1 , Natalia V. Belkova 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.inorgchem.7b03027 Nikolay V. Kireev 1, 2 , Oleg A. Filippov 1 , Alexander A. Pavlov 1 , Lina M. Epstein 1 , Victor D. Makhaev 3 , Victor P. Dyadchenko 2 , Elena S. Shubina 1 , Natalia V. Belkova 1
Affiliation
|
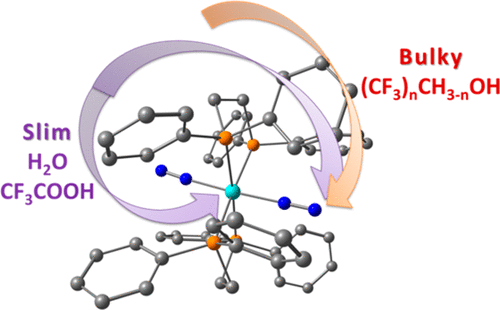
The interaction of trans-W(N2)2(dppe)2 (1; dppe = 1,2-bis(diphenylphosphino)ethane) with relatively weak acids (p-nitrophenol, fluorinated alcohols, CF3COOH) was studied by means of variable temperature IR and NMR spectroscopy and complemented by DFT/B3PW91-D3 calculations. The results show, for the first time, the formation of a hydrogen bond to the coordinated dinitrogen, W–N≡N···H–O, that is preferred over H-bonding to the metal atom, W···H–O, despite the higher proton affinity of the latter. Protonation of the core metal—the undesirable side step in the conversion of N2 to NH3—can be avoided by using weaker and, more importantly, bulkier acids.
中文翻译:

氢键和质子转移至反式W(N 2)2(dppe)2中的立体和酸度控制
通过以下方法研究了反式W(N 2)2(dppe)2(1 ; dppe = 1,2-双(二苯基膦基)乙烷)与相对弱酸(对硝基苯酚,氟化醇,CF 3 COOH)的相互作用DIR / B3PW91-D3计算补充了可变温度IR和NMR光谱。结果首次表明,与配位二氮W–N≡N···H–O形成氢键,这比与金属原子W···H–H的氢键相比更受青睐。 O,尽管后者的质子亲和力更高。芯金属的质子化-N 2转化为NH 3时不希望出现的副步骤-可以通过使用较弱且更重要的是较大体积的酸来避免。
更新日期:2018-01-19
中文翻译:

氢键和质子转移至反式W(N 2)2(dppe)2中的立体和酸度控制
通过以下方法研究了反式W(N 2)2(dppe)2(1 ; dppe = 1,2-双(二苯基膦基)乙烷)与相对弱酸(对硝基苯酚,氟化醇,CF 3 COOH)的相互作用DIR / B3PW91-D3计算补充了可变温度IR和NMR光谱。结果首次表明,与配位二氮W–N≡N···H–O形成氢键,这比与金属原子W···H–H的氢键相比更受青睐。 O,尽管后者的质子亲和力更高。芯金属的质子化-N 2转化为NH 3时不希望出现的副步骤-可以通过使用较弱且更重要的是较大体积的酸来避免。


























 京公网安备 11010802027423号
京公网安备 11010802027423号