当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Large Family of Centrosymmetric and Chiral f-Element-Bearing Iodate Selenates Exhibiting Coordination Number and Dimensional Reductions
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.inorgchem.7b03116 Meiying Qie 1, 2 , Jian Lin 1 , Fang Kong 3 , Mark A. Silver 4 , Zenghui Yue 1, 2 , Xiaomei Wang 1, 2 , Linjuan Zhang 1 , Hongliang Bao 1 , Thomas E. Albrecht-Schmitt 5 , Jian-Qiang Wang 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.inorgchem.7b03116 Meiying Qie 1, 2 , Jian Lin 1 , Fang Kong 3 , Mark A. Silver 4 , Zenghui Yue 1, 2 , Xiaomei Wang 1, 2 , Linjuan Zhang 1 , Hongliang Bao 1 , Thomas E. Albrecht-Schmitt 5 , Jian-Qiang Wang 1
Affiliation
|
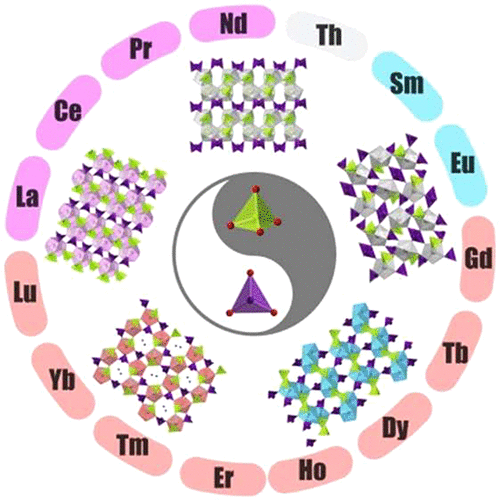
The exploration of phase formation in the f-element-bearing iodate selenate system has resulted in 14 novel rare-earth-containing iodate selenates, Ln(IO3)(SeO4) (Ln = La, Ce, Pr, Nd; LnISeO-1), Ln(IO3)(SeO4)(H2O) (Ln = Sm, Eu; LnISeO-2), and Ln(IO3)(SeO4)(H2O)2·H2O (Ln = Gd, Dy, Ho, Er, Tm, Yb, Lu, Y; LnISeO-3), as well as two new thorium iodate selenates, Th(OH)(IO3)(SeO4)(H2O) (ThISeO-1) and Th(IO3)2(SeO4) (ThISeO-2). LnISeO-3 and ThISeO-2 crystallize in the chiral space group P212121, while LnISeO-1, LnISeO-2, and ThISeO-1 crystallize in the centrosymmetric space group P21/c. The numbers of both coordinating and hydrating water molecules crystallized in LnISeO-1, LnISeO-2, and LnISeO-3 increase along these three series, in line with the increasingly negative values of hydration enthalpies of heavier trivalent lanthanide ions. Such a systematic change in compositions, especially the first coordination sphere of Ln, further induces structural rearrangements, including coordination number and dimensional reductions. More specifically, the structures of LnISeO-1, LnISeO-2, and LnISeO-3 have undergone transitions from 2D Ln–oxo layers with 10-coordinate Ln centers to 1D Ln–oxo chains with 9-coordinate Ln centers and then to 0D Ln–oxo monomers with 8-coordinate Ln centers, respectively. The formation and characterization of this large family of Ln/Th iodate selenates suggest that such a mixed-anion system not only exhibits richer structural chemistry but also can be capable of generating intriguing properties, such as the second-harmonic generation (SHG) effect.
中文翻译:

一大类中心对称和手性含f元素的碘酸盐硒酸盐具有配位数和降维
探索含f元素的碘酸盐硒酸盐体系中的相形成已产生14种新型的含稀土碘酸盐硒酸盐Ln(IO 3)(SeO 4)(Ln = La,Ce,Pr,Nd;LnISeO- 1),Ln(IO 3)(SeO 4)(H 2 O)(Ln = Sm,Eu; LnISeO-2)和Ln(IO 3)(SeO 4)(H 2 O)2 ·H 2 O( Ln = Gd,Dy,Ho,Er,Tm,Yb,Lu,Y; LnISeO-3),以及两种新的碘化亚硒酸亚硒酸盐Th(OH)(IO 3)(SeO 4)(H 2 O)(ThISeO-1)和Th(IO3)2(SeO 4)(ThISeO-2)。LnISeO-3和ThISeO-2在手性空间群P 2 1 2 1 2 1中结晶,而LnISeO-1,LnISeO-2和ThISeO-1在中心对称空间群P 2 1 / c中结晶。在LnISeO-1,LnISeO-2和LnISeO-3中结晶的配位和水合分子的数量沿这三个系列增加,这与较重的三价镧系元素离子的水合焓值越来越负相关。这种组成上的系统变化,尤其是Ln的第一个配位范围,进一步引起结构重排,包括配位数和尺寸的减小。更具体地说,LnISeO-1,LnISeO-2和LnISeO-3的结构分别经历了从具有10个坐标Ln中心的2D Ln-oxo层到具有9个坐标Ln中心的1D Ln-oxo链的转变,然后分别转变为具有8个坐标Ln中心的0D Ln-oxo单体。Ln / Th碘酸盐硒酸盐这一大家族的形成和表征表明,这种混合阴离子体系不仅表现出更丰富的结构化学,而且还能够产生引人入胜的特性,例如二次谐波产生(SHG)效应。
更新日期:2018-01-18
中文翻译:

一大类中心对称和手性含f元素的碘酸盐硒酸盐具有配位数和降维
探索含f元素的碘酸盐硒酸盐体系中的相形成已产生14种新型的含稀土碘酸盐硒酸盐Ln(IO 3)(SeO 4)(Ln = La,Ce,Pr,Nd;LnISeO- 1),Ln(IO 3)(SeO 4)(H 2 O)(Ln = Sm,Eu; LnISeO-2)和Ln(IO 3)(SeO 4)(H 2 O)2 ·H 2 O( Ln = Gd,Dy,Ho,Er,Tm,Yb,Lu,Y; LnISeO-3),以及两种新的碘化亚硒酸亚硒酸盐Th(OH)(IO 3)(SeO 4)(H 2 O)(ThISeO-1)和Th(IO3)2(SeO 4)(ThISeO-2)。LnISeO-3和ThISeO-2在手性空间群P 2 1 2 1 2 1中结晶,而LnISeO-1,LnISeO-2和ThISeO-1在中心对称空间群P 2 1 / c中结晶。在LnISeO-1,LnISeO-2和LnISeO-3中结晶的配位和水合分子的数量沿这三个系列增加,这与较重的三价镧系元素离子的水合焓值越来越负相关。这种组成上的系统变化,尤其是Ln的第一个配位范围,进一步引起结构重排,包括配位数和尺寸的减小。更具体地说,LnISeO-1,LnISeO-2和LnISeO-3的结构分别经历了从具有10个坐标Ln中心的2D Ln-oxo层到具有9个坐标Ln中心的1D Ln-oxo链的转变,然后分别转变为具有8个坐标Ln中心的0D Ln-oxo单体。Ln / Th碘酸盐硒酸盐这一大家族的形成和表征表明,这种混合阴离子体系不仅表现出更丰富的结构化学,而且还能够产生引人入胜的特性,例如二次谐波产生(SHG)效应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号