当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
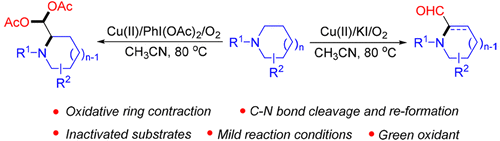
Synthesis of α-Formylated N-Heterocycles and Their 1,1-Diacetates from Inactivated Cyclic Amines Involving an Oxidative Ring Contraction
Organic Letters ( IF 5.2 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.orglett.7b04029 Fang Wang 1 , Yan He 1 , Miaomiao Tian 1 , Xinying Zhang 1 , Xuesen Fan 1
Organic Letters ( IF 5.2 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.orglett.7b04029 Fang Wang 1 , Yan He 1 , Miaomiao Tian 1 , Xinying Zhang 1 , Xuesen Fan 1
Affiliation
|
A novel synthesis of pyrrolidine-2-carbaldehydes or tetrahydropyridine-2-carbaldehydes from the cascade reactions of N-arylpiperidines or N-arylazepanes is presented. Mechanistically, the formation of the title compounds involves an unprecedented oxidative ring contraction of inactivated cyclic amines via Cu(OAc)2/KI/O2-promoted oxidative cleavage and reformation of the C–N bond. Interestingly, when PhI(OAc)2 was used in place of KI, 1,1-diacetates of the corresponding aldehydes were directly obtained with good efficiency. To the best of our knowledge, this is the first example of regioselective C(sp3)–H bond functionalization and C(sp3)–N bond activation of saturated cyclic amines using copper salt and oxygen.
中文翻译:

由涉及氧化环收缩的失活环胺合成α-甲酰化的N-杂环及其1,1-二乙酸酯
提出了一种由N-芳基哌啶或N-芳基氮杂环庚烷的级联反应合成吡咯烷-2-甲醛或四氢吡啶-2-甲醛的新方法。从机理上讲,标题化合物的形成涉及通过Cu(OAc)2 / KI / O 2促进的氧化裂解和C–N键的重整,使灭活的环状胺发生前所未有的氧化环收缩。有趣的是,当用PhI(OAc)2代替KI时,可以高效地直接获得相应醛的1,1-二乙酸酯。据我们所知,这是区域选择性C(sp 3)–H键功能化和C(sp 3)–使用铜盐和氧使饱和环胺的N键活化。
更新日期:2018-01-18
中文翻译:

由涉及氧化环收缩的失活环胺合成α-甲酰化的N-杂环及其1,1-二乙酸酯
提出了一种由N-芳基哌啶或N-芳基氮杂环庚烷的级联反应合成吡咯烷-2-甲醛或四氢吡啶-2-甲醛的新方法。从机理上讲,标题化合物的形成涉及通过Cu(OAc)2 / KI / O 2促进的氧化裂解和C–N键的重整,使灭活的环状胺发生前所未有的氧化环收缩。有趣的是,当用PhI(OAc)2代替KI时,可以高效地直接获得相应醛的1,1-二乙酸酯。据我们所知,这是区域选择性C(sp 3)–H键功能化和C(sp 3)–使用铜盐和氧使饱和环胺的N键活化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号