当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One-pot chemoenzymatic synthesis of trolline and tetrahydroisoquinoline analogues
Chemical Communications ( IF 4.9 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1039/c7cc08024g Jianxiong Zhao 1, 2, 3, 4 , Benjamin R. Lichman 2, 3, 4, 5 , John M. Ward 2, 3, 4, 5 , Helen C. Hailes 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1039/c7cc08024g Jianxiong Zhao 1, 2, 3, 4 , Benjamin R. Lichman 2, 3, 4, 5 , John M. Ward 2, 3, 4, 5 , Helen C. Hailes 1, 2, 3, 4
Affiliation
|
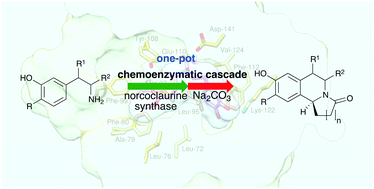
Chemoenzymatic reaction cascades can provide access to chiral compounds from low-cost starting materials in one pot. Here we describe one-pot asymmetric routes to tetrahydroisoquinoline alkaloids (THIAs) using the Pictet–Spenglerase norcoclaurine synthase (NCS) followed by a cyclisation, to give alkaloids with two new heterocyclic rings. These reactions operated with a high atom economy to generate THIAs in high yields.
中文翻译:

一锅化学酶合成的金枪鱼和四氢异喹啉类似物
化学酶促反应级联反应可在一锅中从低成本原料中获得手性化合物。在这里,我们描述了使用Pictet-Spenglerase降鸟嘌呤合酶(NCS)进行一锅法到四氢异喹啉生物碱(THIAs)的一锅不对称路线,然后进行环化反应,得到具有两个新杂环的生物碱。这些反应以高原子经济性进行操作,从而以高收率产生THIA。
更新日期:2018-02-02
中文翻译:

一锅化学酶合成的金枪鱼和四氢异喹啉类似物
化学酶促反应级联反应可在一锅中从低成本原料中获得手性化合物。在这里,我们描述了使用Pictet-Spenglerase降鸟嘌呤合酶(NCS)进行一锅法到四氢异喹啉生物碱(THIAs)的一锅不对称路线,然后进行环化反应,得到具有两个新杂环的生物碱。这些反应以高原子经济性进行操作,从而以高收率产生THIA。


























 京公网安备 11010802027423号
京公网安备 11010802027423号