当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
DNA-Compatible Solid-Phase Combinatorial Synthesis of β-Cyanoacrylamides and Related Electrophiles.
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2018-01-17 , DOI: 10.1021/acscombsci.7b00169 Kevin Pels 1 , Paige Dickson 1 , Hongchan An 1 , Thomas Kodadek 1
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2018-01-17 , DOI: 10.1021/acscombsci.7b00169 Kevin Pels 1 , Paige Dickson 1 , Hongchan An 1 , Thomas Kodadek 1
Affiliation
|
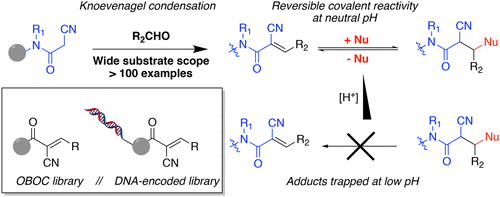
We demonstrate that the Knoevenagel condensation can be exploited in combinatorial synthesis on the solid phase. Condensation products from such reactions were structurally characterized, and their Michael reactivity with thiol and phosphine nucleophiles is described. Cyanoacrylamides were previously reported to react reversibly with thiols, and notably, we show that dilution into low pH buffer can trap covalent adducts, which are isolable via chromatography. Finally, we synthesized both traditional and DNA-encoded one-bead, one-compound libraries containing cyanoacrylamides as a source of cysteine-reactive reversibly covalent protein ligands.
中文翻译:

β-氰基丙烯酰胺和相关亲电试剂的DNA相容固相组合合成。
我们证明了Knoevenagel缩合可以在固相上的组合合成中得到利用。对这类反应的缩合产物进行了结构表征,并描述了它们与硫醇和膦亲核试剂的迈克尔反应性。以前曾报道氰基丙烯酰胺与硫醇可逆反应,并且值得注意的是,我们证明了稀释到低pH缓冲液中可以捕获通过色谱分离的共价加合物。最后,我们合成了传统的和DNA编码的单珠单化合物文库,其中包含氰基丙烯酰胺作为半胱氨酸反应性可逆共价蛋白质配体的来源。
更新日期:2018-01-17
中文翻译:

β-氰基丙烯酰胺和相关亲电试剂的DNA相容固相组合合成。
我们证明了Knoevenagel缩合可以在固相上的组合合成中得到利用。对这类反应的缩合产物进行了结构表征,并描述了它们与硫醇和膦亲核试剂的迈克尔反应性。以前曾报道氰基丙烯酰胺与硫醇可逆反应,并且值得注意的是,我们证明了稀释到低pH缓冲液中可以捕获通过色谱分离的共价加合物。最后,我们合成了传统的和DNA编码的单珠单化合物文库,其中包含氰基丙烯酰胺作为半胱氨酸反应性可逆共价蛋白质配体的来源。


























 京公网安备 11010802027423号
京公网安备 11010802027423号