当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cyclic Peptoids as Topological Templates: Synthesis via Central to Conformational Chirality Induction
Organic Letters ( IF 5.2 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.orglett.7b03786 Assunta D’Amato 1 , Giovanni Pierri 1 , Chiara Costabile 1 , Giorgio Della Sala 1 , Consiglia Tedesco 1 , Irene Izzo 1 , Francesco De Riccardis 1
Organic Letters ( IF 5.2 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.orglett.7b03786 Assunta D’Amato 1 , Giovanni Pierri 1 , Chiara Costabile 1 , Giorgio Della Sala 1 , Consiglia Tedesco 1 , Irene Izzo 1 , Francesco De Riccardis 1
Affiliation
|
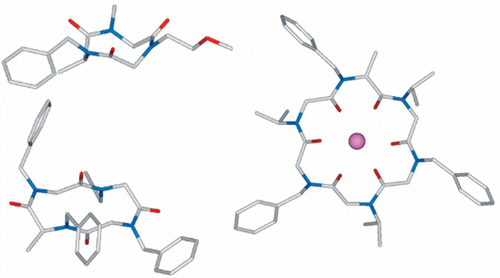
Chiral induction was utilized for the synthesis of diastereopure cyclic peptoids containing an N-benzyl alanine residue. Molecular modeling, NMR spectroscopy, single-crystal X-ray diffraction studies, and HPLC with chiral stationary phase demonstrated easy formation of free and sodium/benzylammonium complexed cyclic oligomers through strategic incorporation of a single stereogenic center in the oligomeric backbone. The synthesis of cyclic peptoids with defined conformational chirality and appropriate side chain topology is now possible.
中文翻译:

环状拟肽作为拓扑模板:通过中心到构象手性诱导合成。
手性诱导用于合成含有N-苄基丙氨酸残基的非对映体环状拟肽。分子建模,NMR光谱学,单晶X射线衍射研究和具有手性固定相的HPLC表明,通过在寡聚主链中策略性地并入单个立体生成中心,可以轻松形成游离的钠盐和苄基铵络合的环状低聚物。现在可以合成具有确定的构象手性和合适的侧链拓扑的环状类肽。
更新日期:2018-01-17
中文翻译:

环状拟肽作为拓扑模板:通过中心到构象手性诱导合成。
手性诱导用于合成含有N-苄基丙氨酸残基的非对映体环状拟肽。分子建模,NMR光谱学,单晶X射线衍射研究和具有手性固定相的HPLC表明,通过在寡聚主链中策略性地并入单个立体生成中心,可以轻松形成游离的钠盐和苄基铵络合的环状低聚物。现在可以合成具有确定的构象手性和合适的侧链拓扑的环状类肽。


























 京公网安备 11010802027423号
京公网安备 11010802027423号