当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular Dynamics Pinpoint the Global Fluorine Effect in Balanoid Binding to PKCε and PKA
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-01-27 00:00:00 , DOI: 10.1021/acs.jcim.7b00504 Ari Hardianto 1 , Fei Liu 1 , Shoba Ranganathan 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-01-27 00:00:00 , DOI: 10.1021/acs.jcim.7b00504 Ari Hardianto 1 , Fei Liu 1 , Shoba Ranganathan 1
Affiliation
|
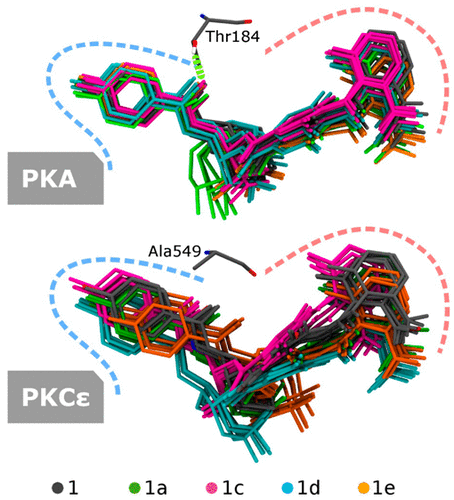
(−)-Balanol is an adenosine triphosphate mimic that inhibits protein kinase C (PKC) isozymes and cAMP-dependent protein kinase (PKA) with limited selectivity. While PKA is known as a tumor promoter, PKC isozymes can be tumor promoters or suppressors. In particular, PKCε is frequently involved in tumorigenesis and a potential target for anticancer drugs. We recently reported that stereospecific fluorination of balanol yielded a balanoid with enhanced selectivity for PKCε over other PKC isozymes and PKA, although the global fluorine effect behind the selectivity enhancement is not fully understood. Interestingly, in contrast to PKA, PKCε is more sensitive to this fluorine effect. Here we investigate the global fluorine effect on the different binding responses of PKCε and PKA to balanoids using molecular dynamics (MD) simulations. For the first time to the best of our knowledge, we found that a structurally equivalent residue in each kinase, Thr184 in PKA and Ala549 in PKCε, is essential for the different binding responses. Furthermore, the study revealed that the invariant Lys, Lys73 in PKA and Lys437 in PKCε, already known to have a crucial role in the catalytic activity of kinases, serves as the main anchor for balanol binding. Overall, while Thr184 in PKA attenuates the effect of fluorination, Ala549 permits remote response of PKCε to fluorine substitution, with implications for rational design of future balanol-based PKCε inhibitors.
中文翻译:

分子动力学查明了总氟在Balanoid与PKCε和PKA结合中的作用
(-)-Balanol是一种三磷酸腺苷模拟物,它以有限的选择性抑制蛋白激酶C(PKC)同工酶和cAMP依赖性蛋白激酶(PKA)。虽然PKA被称为肿瘤启动子,但PKC同工酶可以是肿瘤启动子或抑制剂。特别地,PKCε经常参与肿瘤发生并且是抗癌药物的潜在靶标。我们最近报道说,尽管尚未完全了解选择性增强背后的整体氟效应,但与其他PKC同工酶和PKA相比,Balanol的立体定向氟化产生了对PKCε选择性增强的黄酮类化合物。有趣的是,与PKA相比,PKCε对这种氟效应更敏感。在这里,我们使用分子动力学(MD)模拟研究了氟对PKCε和PKA对类胡萝卜素的不同结合反应的整体氟效应。就我们所知,这是第一次,我们发现每种激酶的结构上相同的残基,即PKA中的Thr184和PKCε中的Ala549,对于不同的结合反应至关重要。此外,研究表明,已知在激酶的催化活性中具有关键作用的不变的Lys,PKA中的Lys73和PKCε中的Lys437,充当了Balanol结合的主要锚点。总体而言,尽管PKA中的Thr184减弱了氟化作用,但Ala549允许PKCε对氟取代的远程响应,这对未来基于Balanol的PKCε抑制剂的合理设计产生了影响。已知在激酶的催化活性中起关键作用的是PKA中的Lys73和PKCε中的Lys437,它们是巴拉诺醇结合的主要锚点。总体而言,尽管PKA中的Thr184减弱了氟化作用,但Ala549允许PKCε对氟取代的远程响应,这对未来基于Balanol的PKCε抑制剂的合理设计产生了影响。已知在激酶的催化活性中起关键作用的是PKA中的Lys73和PKCε中的Lys437,它们是巴拉诺醇结合的主要锚点。总体而言,尽管PKA中的Thr184减弱了氟化作用,但Ala549允许PKCε对氟取代的远程响应,这对未来基于Balanol的PKCε抑制剂的合理设计产生了影响。
更新日期:2018-01-27
中文翻译:

分子动力学查明了总氟在Balanoid与PKCε和PKA结合中的作用
(-)-Balanol是一种三磷酸腺苷模拟物,它以有限的选择性抑制蛋白激酶C(PKC)同工酶和cAMP依赖性蛋白激酶(PKA)。虽然PKA被称为肿瘤启动子,但PKC同工酶可以是肿瘤启动子或抑制剂。特别地,PKCε经常参与肿瘤发生并且是抗癌药物的潜在靶标。我们最近报道说,尽管尚未完全了解选择性增强背后的整体氟效应,但与其他PKC同工酶和PKA相比,Balanol的立体定向氟化产生了对PKCε选择性增强的黄酮类化合物。有趣的是,与PKA相比,PKCε对这种氟效应更敏感。在这里,我们使用分子动力学(MD)模拟研究了氟对PKCε和PKA对类胡萝卜素的不同结合反应的整体氟效应。就我们所知,这是第一次,我们发现每种激酶的结构上相同的残基,即PKA中的Thr184和PKCε中的Ala549,对于不同的结合反应至关重要。此外,研究表明,已知在激酶的催化活性中具有关键作用的不变的Lys,PKA中的Lys73和PKCε中的Lys437,充当了Balanol结合的主要锚点。总体而言,尽管PKA中的Thr184减弱了氟化作用,但Ala549允许PKCε对氟取代的远程响应,这对未来基于Balanol的PKCε抑制剂的合理设计产生了影响。已知在激酶的催化活性中起关键作用的是PKA中的Lys73和PKCε中的Lys437,它们是巴拉诺醇结合的主要锚点。总体而言,尽管PKA中的Thr184减弱了氟化作用,但Ala549允许PKCε对氟取代的远程响应,这对未来基于Balanol的PKCε抑制剂的合理设计产生了影响。已知在激酶的催化活性中起关键作用的是PKA中的Lys73和PKCε中的Lys437,它们是巴拉诺醇结合的主要锚点。总体而言,尽管PKA中的Thr184减弱了氟化作用,但Ala549允许PKCε对氟取代的远程响应,这对未来基于Balanol的PKCε抑制剂的合理设计产生了影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号