Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Progress in clinical research in surgery and IDEAL.
The Lancet ( IF 168.9 ) Pub Date : 2018-07-07 , DOI: 10.1016/s0140-6736(18)30102-8 Peter McCulloch 1 , Joshua Feinberg 2 , Yiannis Philippou 1 , Angelos Kolias 3 , Sean Kehoe 4 , Gillian Lancaster 5 , Jenny Donovan 6 , Tatjana Petrinic 7 , Riaz Agha 8 , Christopher Pennell 2
The Lancet ( IF 168.9 ) Pub Date : 2018-07-07 , DOI: 10.1016/s0140-6736(18)30102-8 Peter McCulloch 1 , Joshua Feinberg 2 , Yiannis Philippou 1 , Angelos Kolias 3 , Sean Kehoe 4 , Gillian Lancaster 5 , Jenny Donovan 6 , Tatjana Petrinic 7 , Riaz Agha 8 , Christopher Pennell 2
Affiliation
|
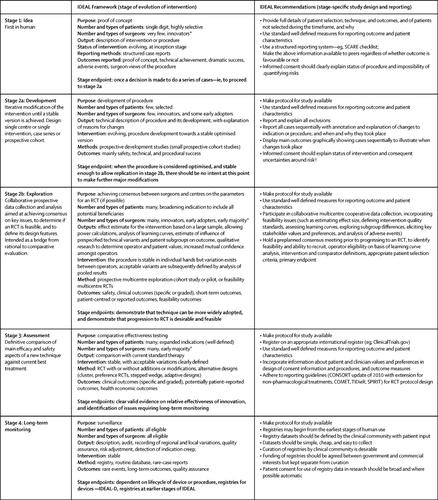
The quality of clinical research in surgery has long attracted criticism. High-quality randomised trials have proved difficult to undertake in surgery, and many surgical treatments have therefore been adopted without adequate supporting evidence of efficacy and safety. This evidence deficit can adversely affect research funding and reimbursement decisions, lead to slow adoption of innovations, and permit widespread adoption of procedures that offer no benefit, or cause harm. Improvement in the quality of surgical evidence would therefore be valuable. The Idea, Development, Exploration, Assessment, and Long-term Follow-up (IDEAL) Framework and Recommendations specify desirable qualities for surgical studies, and outline an integrated evaluation pathway for surgery, and similar complex interventions. We used the IDEAL Recommendations to assess methodological progress in surgical research over time, assessed the uptake and influence of IDEAL, and identified the challenges to further methodological progress. Comparing studies from the periods 2000-04 and 2010-14, we noted apparent improvement in the use of standard outcome measures, adoption of Consolidated Standards of Reporting Trials (CONSORT) standards, and assessment of the quality of surgery and of learning curves, but no progress in the use of qualitative research or reporting of modifications during procedure development. Better education about research, integration of rigorous evaluation into routine practice and training, and linkage of such work to awards systems could foster further improvements in surgical evidence. IDEAL has probably contributed only slightly to the improvements described to date, but its uptake is accelerating rapidly. The need for the integrated evaluation template IDEAL offers for surgery and other complex treatments is becoming more widely accepted.
中文翻译:

外科和IDEAL的临床研究进展。
长期以来,外科临床研究的质量一直受到批评。高质量的随机试验已证明很难在手术中进行,因此许多手术治疗在没有足够的疗效和安全性证据支持的情况下被采用。这种证据不足会对研究资助和报销决定产生不利影响,导致创新采用缓慢,并允许广泛采用无益或造成伤害的程序。因此,提高手术证据的质量将是有价值的。理念、发展、探索、评估和长期随访 (IDEAL) 框架和建议规定了外科研究的理想质量,并概述了外科手术和类似复杂干预措施的综合评估途径。我们使用 IDEAL 建议来评估随着时间的推移外科研究的方法学进展,评估 IDEAL 的采用和影响,并确定进一步方法学进展的挑战。比较 2000-04 年和 2010-14 年期间的研究,我们注意到标准结果测量的使用、报告试验综合标准 (CONSORT) 标准的采用以及手术质量和学习曲线评估方面的明显改善,但在程序开发过程中使用定性研究或修改报告没有进展。更好的研究教育、将严格的评估纳入日常实践和培训,以及将此类工作与奖励制度联系起来,可以促进外科证据的进一步改进。IDEAL 可能对迄今为止所描述的改进贡献很小,但它的采用正在迅速加速。IDEAL 为手术和其他复杂治疗提供的综合评估模板的需求正变得越来越广泛。
更新日期:2018-07-08
中文翻译:

外科和IDEAL的临床研究进展。
长期以来,外科临床研究的质量一直受到批评。高质量的随机试验已证明很难在手术中进行,因此许多手术治疗在没有足够的疗效和安全性证据支持的情况下被采用。这种证据不足会对研究资助和报销决定产生不利影响,导致创新采用缓慢,并允许广泛采用无益或造成伤害的程序。因此,提高手术证据的质量将是有价值的。理念、发展、探索、评估和长期随访 (IDEAL) 框架和建议规定了外科研究的理想质量,并概述了外科手术和类似复杂干预措施的综合评估途径。我们使用 IDEAL 建议来评估随着时间的推移外科研究的方法学进展,评估 IDEAL 的采用和影响,并确定进一步方法学进展的挑战。比较 2000-04 年和 2010-14 年期间的研究,我们注意到标准结果测量的使用、报告试验综合标准 (CONSORT) 标准的采用以及手术质量和学习曲线评估方面的明显改善,但在程序开发过程中使用定性研究或修改报告没有进展。更好的研究教育、将严格的评估纳入日常实践和培训,以及将此类工作与奖励制度联系起来,可以促进外科证据的进一步改进。IDEAL 可能对迄今为止所描述的改进贡献很小,但它的采用正在迅速加速。IDEAL 为手术和其他复杂治疗提供的综合评估模板的需求正变得越来越广泛。



























 京公网安备 11010802027423号
京公网安备 11010802027423号