PLOS ONE ( IF 3.7 ) Pub Date : 2018-01-16 , DOI: 10.1371/journal.pone.0191282 Vitor M. Almeida , Maira A. Frutuoso , Sandro R. Marana
|
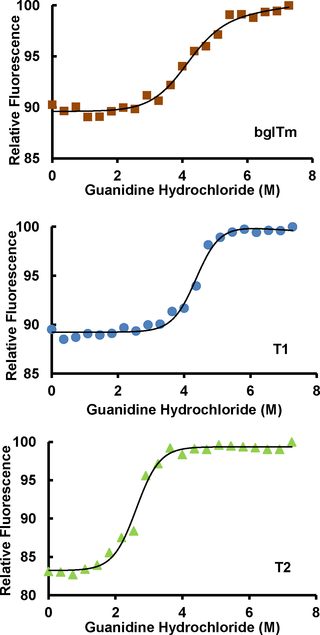
Proteins that fold as (β/α)8 barrels are thought to have evolved from half-barrels that underwent duplication and fusion events. The evidence is particularly clear for small barrels, which have almost identical halves. Additionally, computational calculations of the thermodynamic stability of these structures in the presence of denaturants have revealed that (β/α)8 barrels contain two subunits or domains corresponding to half-barrels. Hence, within (β/α)8 barrels, half-barrels are self-contained units. Here, we tested this hypothesis using β-glucosidase from the bacterium Thermotoga maritima (bglTm), which has a (β/α)8 barrel structure. Mutations were introduced to disrupt the noncovalent contacts between its halves and reveal the presence of two domains within bglTm, thus resulting in the creation of mutants T1 (containing W12A and I217A mutations) and T2 (containing W12A, H195A, I217A and F404A mutations). Mutants T1 and T2 were properly folded, as indicated by their fluorescence spectra and enzyme kinetic parameters. T1 and wild-type bglTm were equally stable, as shown by the results of thermal inactivation, differential scanning fluorimetry and guanidine hydrochloride denaturation experiments. However, T2 showed a first-order inactivation at 80°C, a single melting temperature of 82°C and only one transition concentration (c50) in 2.4 M guanidine hydrochloride. Additionally, T1 and T2 exhibited a cooperative denaturation process that followed a two-state model (m-values equal to 1.4 and 1.6 kcal/mol/M, respectively), similar to that of wild-type bglTm (1.2 kcal/mol/M). Hence, T1 and T2 each denatured as a single unit, although they contained different degrees of disruption between their halves. In conclusion, bglTm halves are equivalent in terms of their thermal and chemical stability; thus, their separate contributions to (β/α)8 barrel unfolding cannot be disentangled.
中文翻译:

在(β/α)8桶β-葡萄糖苷酶中搜索独立的(β/α)4个子域
折叠成(β/α)8桶的蛋白质被认为是从经历了复制和融合事件的半桶进化而来的。对于桶几乎完全相同的小桶,证据尤为明显。另外,在存在变性剂的情况下,这些结构的热力学稳定性的计算结果表明,(β/α)8桶包含两个与半桶相对应的亚基或区域。因此,在(β/α)8桶内,半桶是独立的单位。在这里,我们使用来自海洋嗜热菌(bglTm)的β-葡萄糖苷酶(b / l)8验证了该假设桶形结构。引入突变以破坏其两半之间的非共价接触并揭示bglTm中两个结构域的存在,从而导致产生突变体T1(包含W12A和I217A突变)和T2(包含W12A,H195A,I217A和F404A突变)。如突变体T1和T2的荧光光谱和酶动力学参数所示,它们被正确折叠。T1和野生型bglTm具有相同的稳定性,如热灭活,差示扫描荧光法和盐酸胍变性实验的结果所示。然而,T2在80°C时表现出一阶失活,单个熔化温度为82°C,并且只有一个转变浓度(c 50)在2.4 M盐酸胍中。另外,T1和T2表现出遵循两个状态模型(m值分别等于1.4和1.6 kcal / mol / M)的协同变性过程,类似于野生型bglTm(1.2 kcal / mol / M)。 )。因此,尽管T1和T2在它们的两半之间包含不同程度的破坏,但它们各自都被变性为一个单元。总之,就热稳定性和化学稳定性而言,bglTm一半相等。因此,它们对(β/α)8桶展开的单独贡献无法解开。



























 京公网安备 11010802027423号
京公网安备 11010802027423号