Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2018-01-16 , DOI: 10.1016/j.jinorgbio.2018.01.005 Anna Bielenica , Aleksandra Drzewiecka-Antonik , Paweł Rejmak , Joanna Stefańska , Michał Koliński , Sebastian Kmiecik , Bogdan Lesyng , Marta Włodarczyk , Piotr Pietrzyk , Marta Struga
|
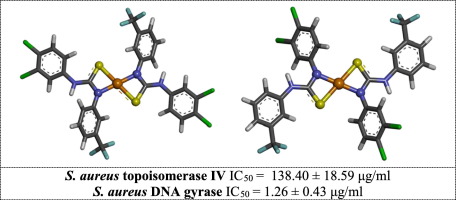
A series of Cu(II) complexes of 3-(trifluoromethyl)phenylthiourea derivatives was synthesized. Their structural properties were investigated by spectroscopic techniques (infrared and electron paramagnetic resonance), as well as molecular modeling. All studied coordination compounds are mononuclear complexes containing two chelating ligands bonded to the metal cation via S and deprotonated N atoms. The new chelates were evaluated for their antimicrobial potency. The complex of 1-(3,4-dichlorophenyl)-3-[3-(trifluoromethyl)phenyl]thiourea (3) presented the highest activity against Gram-positive pathogens, even stronger than the activity of its non-complexed counterpart and the reference drug. The compound also prevented the biofilm formation of methicillin-resistant and standard strains of staphylococcal cocci. The title derivatives were found to be effective inhibitors of DNA gyrase and topoisomerase IV isolated from Staphylococcus aureus. The binding modes of the ligand L3 with DNA gyrase and topoisomerase IV were presented.
中文翻译:

1,3-二取代硫脲配体的II型拓扑异构酶靶向的铜(II)配合物的合成,结构和抗菌研究
合成了一系列的3-(三氟甲基)苯基硫脲衍生物的Cu(II)配合物。通过光谱技术(红外和电子顺磁共振)以及分子模型研究了它们的结构性质。所有研究的配位化合物都是单核络合物,包含两个通过S和去质子化的N键与金属阳离子键合的螯合配体。对新的螯合物的抗菌效力进行了评估。1-(3,4-二氯苯基)-3- [3-(三氟甲基)苯基]硫脲的络合物(3)表现出对革兰氏阳性病原体最高的活性,甚至比其非复杂对应物和参考药物的活性还要强。该化合物还阻止了耐甲氧西林和标准葡萄球菌的菌株的生物膜形成。发现标题衍生物是从金黄色葡萄球菌中分离的DNA促旋酶和拓扑异构酶IV的有效抑制剂。提出了配体L3与DNA促旋酶和拓扑异构酶IV的结合模式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号