当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metal-Free and Selective Oxidation of Furfural to Furoic Acid with an N-Heterocyclic Carbene Catalyst
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acssuschemeng.7b03681 Navneet Kumar Gupta 1, 2 , Atsushi Fukuoka 1 , Kiyotaka Nakajima 1, 3
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acssuschemeng.7b03681 Navneet Kumar Gupta 1, 2 , Atsushi Fukuoka 1 , Kiyotaka Nakajima 1, 3
Affiliation
|
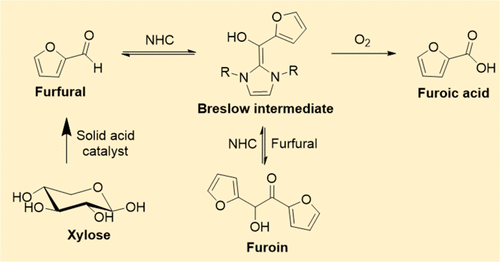
Aerobic oxidation of biomass-derived furfural to furoic acid was studied with an N-heterocyclic carbene as a homogeneous catalyst. Carbene species generated in situ on 1,3-bis(2,4,6-trimethylphenyl) imidazolium chloride with a strong organic base (1,8-diazabicyclo[5.4.0]undec-7-ene) was highly active and selective for the formation of furoic acid in dimethyl sulfoxide at 40 °C. This reaction initiates the formation of a Breslow intermediate between an N-heterocyclic carbene and a furfural molecule and the subsequent activation of molecular O2. While the active carbene catalyst promoted furfural dimerization to afford furoin as a side reaction, furoin was decomposed into the Breslow intermediate and furfural through a reverse reaction, which were then converted quantitatively to furoic acid. Kinetic studies revealed that the apparent activation energy for this furfural oxidation was only 20 kJ mol–1, which is significantly lower than that with a supported Au catalyst (30.4 kJ mol–1). The N-heterocyclic carbene catalyst can oxidize various furan-based aldehydes with high selectivity; however, the electron-withdrawing group bonded to the furan ring has a negative effect on the reaction rate. Furfural can also be oxidized selectively to furoic acid, even in the presence of byproducts that are formed by acid-catalyzed dehydration of xylose with Amberlyst-70. As a result, a sequential reaction system based on initial dehydration and subsequent aerobic oxidation was developed for the production of furoic acid from xylose, without the need for furfural purification, using Amberlyst-70 (a solid acid) and an N-heterocyclic carbene catalyst.
中文翻译:

N-杂环碳烯催化剂对糠醛的无金属选择性氧化为糠酸
以N-杂环卡宾为均相催化剂,研究了生物质衍生糠醛的好氧氧化为糠酸。在具有强有机碱(1,8-二氮杂双环[5.4.0] undec-7-ene)的1,3-双(2,4,6-三甲基苯基)咪唑鎓氯化物上原位产生的碳氢化合物具有很高的活性和选择性40°C下在二甲基亚砜中形成糠酸。该反应引发N-杂环卡宾与糠醛分子之间的Breslow中间体的形成以及随后的分子O 2的活化。。尽管活性卡宾催化剂促进糠醛二聚化以提供副反应的糠醛,但糠醛通过逆反应分解为Breslow中间体和糠醛,然后定量转化为糠酸。动力学研究表明,糠醛氧化的表观活化能仅为20 kJ mol –1,明显低于负载型Au催化剂(30.4 kJ mol –1)的活化能。)。N-杂环卡宾催化剂可以高选择性地氧化各种呋喃基醛。然而,结合在呋喃环上的吸电子基团对反应速率有负面影响。糠醛也可以选择性地氧化为糠酸,即使在副产物的存在下,副产物是由木糖用Amberlyst-70酸催化脱水形成的。结果,开发了一种基于初始脱水和随后的好氧氧化的顺序反应系统,用于使用Amberlyst-70(固体酸)和N-杂环卡宾催化剂从木糖生产糠酸,而无需糠醛纯化。 。
更新日期:2018-01-17
中文翻译:

N-杂环碳烯催化剂对糠醛的无金属选择性氧化为糠酸
以N-杂环卡宾为均相催化剂,研究了生物质衍生糠醛的好氧氧化为糠酸。在具有强有机碱(1,8-二氮杂双环[5.4.0] undec-7-ene)的1,3-双(2,4,6-三甲基苯基)咪唑鎓氯化物上原位产生的碳氢化合物具有很高的活性和选择性40°C下在二甲基亚砜中形成糠酸。该反应引发N-杂环卡宾与糠醛分子之间的Breslow中间体的形成以及随后的分子O 2的活化。。尽管活性卡宾催化剂促进糠醛二聚化以提供副反应的糠醛,但糠醛通过逆反应分解为Breslow中间体和糠醛,然后定量转化为糠酸。动力学研究表明,糠醛氧化的表观活化能仅为20 kJ mol –1,明显低于负载型Au催化剂(30.4 kJ mol –1)的活化能。)。N-杂环卡宾催化剂可以高选择性地氧化各种呋喃基醛。然而,结合在呋喃环上的吸电子基团对反应速率有负面影响。糠醛也可以选择性地氧化为糠酸,即使在副产物的存在下,副产物是由木糖用Amberlyst-70酸催化脱水形成的。结果,开发了一种基于初始脱水和随后的好氧氧化的顺序反应系统,用于使用Amberlyst-70(固体酸)和N-杂环卡宾催化剂从木糖生产糠酸,而无需糠醛纯化。 。


























 京公网安备 11010802027423号
京公网安备 11010802027423号