Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-01-16 , DOI: 10.1016/j.bioorg.2017.12.022 Madiha Kazmi , Sumera Zaib , Aliya Ibrar , Sayyeda Tayyeba Amjad , Zainab Shafique , Saifullah Mehsud , Aamer Saeed , Jamshed Iqbal , Imtiaz Khan
|
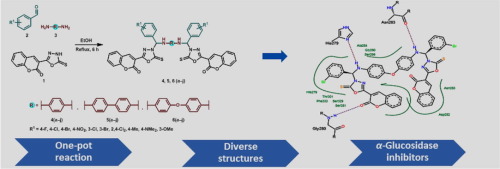
Diabetes mellitus (DM), a chronic multifarious metabolic disorder resulting from impaired glucose homeostasis has become one of the most challenging diseases with severe life threat to public health. The inhibition of α-glucosidase, a key carbohydrate hydrolyzing enzyme, could serve as one of the effective methodology in both preventing and treating diabetes through controlling the postprandial glucose levels and suppressing postprandial hyperglycemia. In this context, three series of diamine-bridged bis-coumarinyl oxadiazole conjugates were designed and synthesized by one-pot multi-component methodology. The synthesized conjugates (4a–j, 5a–j, 6a–j) were evaluated as potential inhibitors of glucosidases. Compound 6f containing 4,4′-oxydianiline linker was identified as the lead and selective inhibitor of α-glucosidase enzyme with an IC50 value of 0.07 ± 0.001 μM (acarbose: IC50 = 38.2 ± 0.12 μM). This inhibition efficacy was ∼545-fold higher compared to the standard drug. Compound 6f was also emerged as the lead molecule against intestinal maltase-glucoamylase with good inhibition strength (IC50 = 0.04 ± 0.02 μM) compared to acarbose (IC50 = 0.06 ± 0.01 μM). Against β-glucosidase enzyme, compound 6 g was noted as the lead inhibitor with IC50 value of 0.08 ± 0.002 μM. Michaelis–Menten kinetic experiments were performed to explore the mechanism of inhibition. Molecular docking studies of the synthesized library of hybrid structures against glucosidase enzyme were performed to describe ligand-protein interactions at molecular level that provided an insight into the biological properties of the analyzed compounds. The results suggested that the inhibitors could be stabilized in the active site through the formation of multiple interactions with catalytic residues in a cooperative fashion. In addition, strong binding interactions of the compounds with the amino acid residues were effective for the successful identification of α-glucosidase inhibitors.
中文翻译:

α-葡萄糖苷酶抑制剂作为2型糖尿病有效治疗剂的产品组合的新进入:二胺桥接香豆素基恶二唑共轭物的设计,生物评价和一锅多组分合成
糖尿病(DM)是一种由葡萄糖稳态失衡引起的慢性多发性代谢疾病,已成为最具挑战性的疾病之一,对公共健康造成严重的生命威胁。抑制α葡糖苷酶,一键碳水化合物水解酶,可以通过控制餐后葡萄糖水平,并抑制餐后高血糖作为在两个预防和治疗糖尿病的有效方法之一。在这种情况下,通过一锅多组分方法设计和合成了三系列的二胺桥联的双-香豆基恶二唑共轭物。合成的结合物(4a–j,5a–j,6a–j)被评估为糖苷酶的潜在抑制剂。化合物6f含有4,4'-氧化二苯胺连接基的化合物被鉴定为α-葡萄糖苷酶的先导和选择性抑制剂,IC 50值为0.07±0.001μM(阿卡波糖:IC 50 = 38.2±0.12μM)。与标准药物相比,该抑制效力高约545倍。 与阿卡波糖(IC 50 = 0.06± 0.01μM)相比,化合物6f还作为抗肠麦芽糖酶-葡糖淀粉酶的先导分子出现,具有良好的抑制强度(IC 50 = 0.04± 0.02μM)。针对β-葡萄糖苷酶,化合物6 g被认为是IC 50的主要抑制剂值为0.08±0.002μM。进行了Michaelis-Menten动力学实验以探索抑制作用的机理。进行了针对葡萄糖苷酶的杂化结构合成文库的分子对接研究,以描述分子水平上的配体-蛋白质相互作用,从而提供了对所分析化合物生物学特性的了解。结果表明,抑制剂可以通过与催化残基以协作方式形成多次相互作用而稳定在活性位点。另外,化合物与氨基酸残基的强结合相互作用对于成功鉴定α-葡萄糖苷酶抑制剂是有效的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号