European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2018-01-16 , DOI: 10.1016/j.ejmech.2018.01.034 Wei Tuo , Mélanie Bollier , Natascha Leleu-Chavain , Lucas Lemaire , Amélie Barczyk , Xavier Dezitter , Frédérique Klupsch , Fabien Szczepanski , John Spencer , Philippe Chavatte , Régis Millet
|
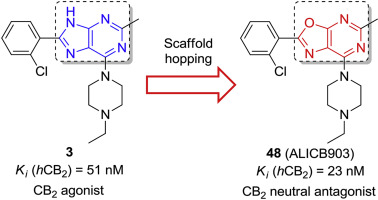
A series of novel oxazolo[5,4-d]pyrimidines was designed via a scaffold hopping strategy and synthesized through a newly developed approach. All these compounds were evaluated for their biological activity toward CB1/CB2 cannabinoid receptors, their metabolic stability in mice liver microsomes and their cytotoxicity against several cell lines. Eight compounds have been identified as CB2 ligands with Ki values less than 1 μM. It is noteworthy that 2-(2-chlorophenyl)-5-methyl-7-(4-methylpiperazin-1-yl) oxazolo[5,4-d]pyrimidine 47 and 2-(2-chlorophenyl)-7-(4-ethylpiperazin-1-yl)- 5-methyloxazolo[5,4-d]pyrimidine 48 showed CB2 binding affinity in the nanomolar range and significant selectivity over CB1 receptors. Interestingly, functionality studies imply that they behave as competitive neutral antagonists. Moreover, all tested compounds are devoid of cytotoxicity toward several cell lines, including Chinese hamster ovary cells (CHO) and human colorectal adenocarcinoma cells HT29.



























 京公网安备 11010802027423号
京公网安备 11010802027423号