当前位置:
X-MOL 学术
›
Macromol. Rapid Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Polypeptide Polymer Brushes by Light‐Induced Surface Polymerization of Amino Acid N‐Carboxyanhydrides
Macromolecular Rapid Communications ( IF 4.6 ) Pub Date : 2018-01-15 , DOI: 10.1002/marc.201700743 Timo Stukenkemper 1 , Xavier Paquez 2 , M. W. G. M. Verhoeven 3 , Emiel J. M. Hensen 3 , Aylvin A. Dias 2 , Dermot F. Brougham 4 , Andreas Heise 5
Macromolecular Rapid Communications ( IF 4.6 ) Pub Date : 2018-01-15 , DOI: 10.1002/marc.201700743 Timo Stukenkemper 1 , Xavier Paquez 2 , M. W. G. M. Verhoeven 3 , Emiel J. M. Hensen 3 , Aylvin A. Dias 2 , Dermot F. Brougham 4 , Andreas Heise 5
Affiliation
|
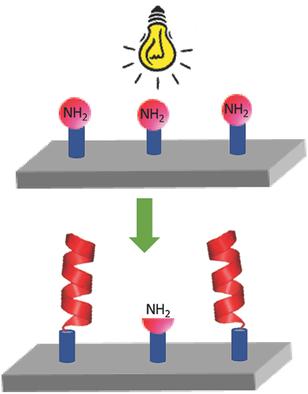
Silicon wafers are decorated with photoamine generator 4,5‐dimethoxy‐2‐nitrobenzyl 3‐(triethoxysilyl)propyl carbamate. UV‐irradiation in the presence of benzyl‐l‐glutamate N‐carboxyanhydride is carried out, resulting in the release of the surface‐bound primary amines, making them viable N‐carboxyanhydride (NCA) polymerization initiators. Successful polypeptide grafting is confirmed by water contact angle measurements as well as by ellipsometry, revealing a poly(benzyl‐l‐glutamate) (PBLG) layer of ≈3 nm. X‐ray photoelectron spectroscopy confirms the presence of amide groups in the grafted PBLG while time‐of‐flight secondary ion mass spectroscopy provides additional evidence for the presence of PBLG on the surface. Evaluation of negative control samples confirms successful UV surface grafting. The approach is thus established as a viable general method for light exposure directable polypeptide functionalization of silicon surfaces.
中文翻译:

氨基酸N-羧酸盐的光诱导表面聚合的多肽聚合物刷
硅片上装饰有光胺生成剂4,5-二甲氧基-2-硝基苄基3-(三乙氧基甲硅烷基)丙基氨基甲酸酯。UV线照射在苄基的存在下升-谷氨酸Ñ -carboxyanhydride被执行,从而导致表面结合的伯胺的释放,使得它们可行Ñ -carboxyanhydride(NCA)聚合引发剂。成功的多肽接枝是通过水接触角的测量,以及通过椭圆光度法证实,揭示聚(苄基-升谷氨酸盐(PBLG)层约3 nm。X射线光电子能谱证实了接枝的PBLG中存在酰胺基,而飞行时间二次离子质谱则提供了表面上存在PBLG的其他证据。阴性对照样品的评估证实了成功的紫外线表面接枝。因此,该方法被确立为用于硅表面的受光定向的多肽功能化的可行的通用方法。
更新日期:2018-01-15
中文翻译:

氨基酸N-羧酸盐的光诱导表面聚合的多肽聚合物刷
硅片上装饰有光胺生成剂4,5-二甲氧基-2-硝基苄基3-(三乙氧基甲硅烷基)丙基氨基甲酸酯。UV线照射在苄基的存在下升-谷氨酸Ñ -carboxyanhydride被执行,从而导致表面结合的伯胺的释放,使得它们可行Ñ -carboxyanhydride(NCA)聚合引发剂。成功的多肽接枝是通过水接触角的测量,以及通过椭圆光度法证实,揭示聚(苄基-升谷氨酸盐(PBLG)层约3 nm。X射线光电子能谱证实了接枝的PBLG中存在酰胺基,而飞行时间二次离子质谱则提供了表面上存在PBLG的其他证据。阴性对照样品的评估证实了成功的紫外线表面接枝。因此,该方法被确立为用于硅表面的受光定向的多肽功能化的可行的通用方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号