Electrochimica Acta ( IF 6.6 ) Pub Date : 2018-01-13 , DOI: 10.1016/j.electacta.2018.01.079 Ozge Turkay , Sibel Barışçı , Ebru Ulusoy , Mine Gül Şeker , Anatoli Dimoglo
|
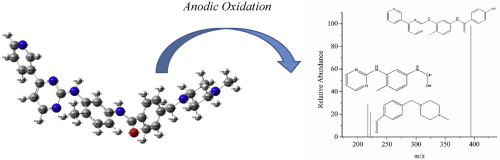
Degradation of anti-cancer drug Imatinib (IMT) in aqueous solution by anodic oxidation at different electrodes, namely Ti/RuO2, Ti/Pt, Ti/IrO2RuO2, Ti/Ta2O5
SnO2
IrO2, Ti/IrO2
Ta2O5 SnO2/Pt and boron doped diamond (BDD), has been investigated. The effect of the applied current density and initial pH of the solution were examined. The electron structure calculations has been calculated by density functional theory (DFT). These results were compared to the results of liquid chromatography-mass spectroscopy (LC-MS-MS) and the metabolites has been identified. Moreover, the toxicity behavior of treated IMT solution by Ti/RuO2 and BDD electrodes were clarified and compared. IMT degradation was in the order of Ti/RuO2>Ti/Pt > Ti/IrO2
Ta2O5>Ti/Ta2O5
SnO2
IrO2>Ti/IrO2
RuO2>SnO2/Pt > BDD. At the initial stage of the IMT degradation, fragments with molecular weights 394 and 100 g mol−1 are formed. As a result of further degradation, the metabolites with m/z = 394 g mol−1 and m/z = 222 g mol−1 were formed. The bond
NH
C/
O breakup may happen with the appearance of fragments having molecular weights 217 and 277 g mol−1. Experimental exploration of the metabolites has been also confirmed by the LC-MS-MS. EC50 value of IMT solution was determined as 2.50 mg L−1. Toxicity of BDD treated solution was significantly higher than Ti/RuO2 treated solution at the end of the process. This study demonstrates that anodic oxidation of IMT could be applied for the degradation of such anti-cancer drugs in wastewater treatment. Considering the toxicity behavior of treated IMT solutions, the usage of Ti/RuO2 as anode was the most effective one together with its oxidation capacity.
中文翻译:

抗癌药物伊马替尼在不同电极上的阳极氧化:动力学,转化副产物和毒性评估
Ti / RuO 2,Ti / Pt,Ti / IrO 2 RuO 2,Ti / Ta 2 O 5
SnO 2
IrO 2,Ti / IrO 2
Ta 2 O 5 SnO 2已经研究了/ Pt和硼掺杂的金刚石(BDD)。检查了施加的电流密度和溶液初始pH的影响。电子结构计算已通过密度泛函理论(DFT)进行了计算。将这些结果与液相色谱-质谱(LC-MS-MS)的结果进行了比较,并鉴定了代谢物。此外,澄清并比较了Ti / RuO 2和BDD电极处理的IMT溶液的毒性行为。IMT降解的顺序为Ti / RuO 2 > Ti / Pt> Ti / IrO 2
Ta 2 O 5 > Ti / Ta 2 O 5
SnO 2
IrO 2 > Ti / IrO 2
RuO2 > SnO 2 / Pt> BDD。在IMT降解的初始阶段,形成分子量为394和100 g mol -1的片段。作为进一步降解的结果,形成了具有m / z = 394g mol -1和m / z = 222g mol -1的代谢物。随着分子量为217和277 g mol -1的片段的出现,可能会发生
NH
C /
O键断裂。LC-MS-MS也证实了对代谢产物的实验探索。IMT溶液的EC 50值确定为2.50 mg L -1。在处理结束时,BDD处理过的溶液的毒性明显高于Ti / RuO 2处理过的溶液。这项研究表明,IMT的阳极氧化可用于废水处理中此类抗癌药物的降解。考虑到处理过的IMT溶液的毒性行为,以Ti / RuO 2作为阳极是最有效的方法,同时还具有氧化能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号